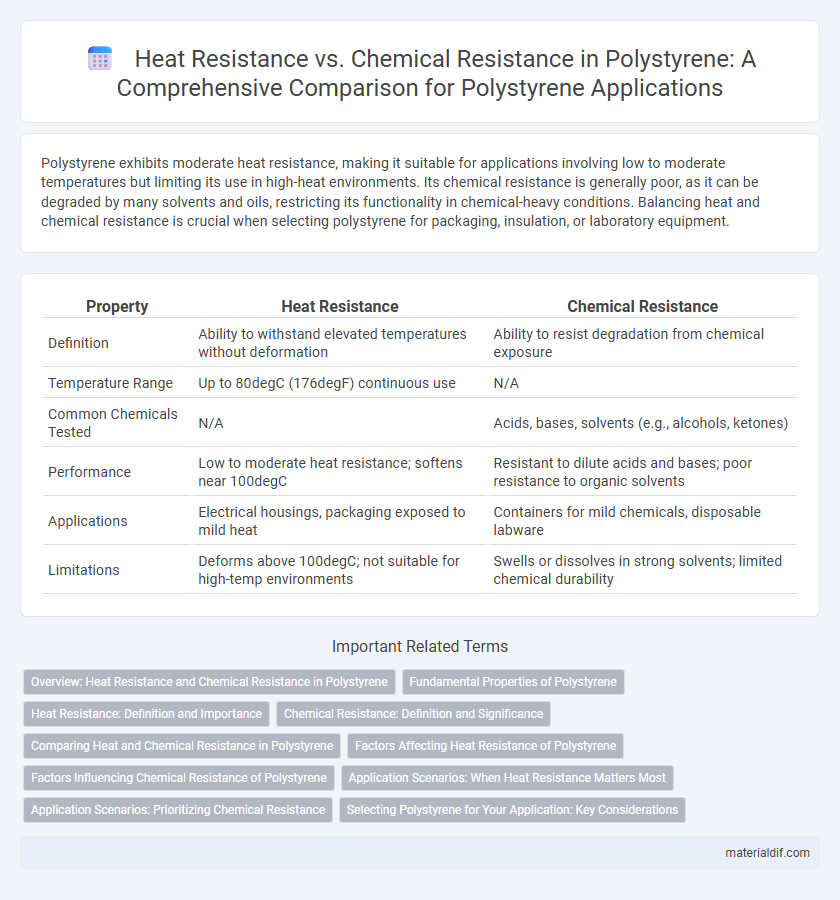
Polystyrene exhibits moderate heat resistance, making it suitable for applications involving low to moderate temperatures but limiting its use in high-heat environments. Its chemical resistance is generally poor, as it can be degraded by many solvents and oils, restricting its functionality in chemical-heavy conditions. Balancing heat and chemical resistance is crucial when selecting polystyrene for packaging, insulation, or laboratory equipment.
Table of Comparison
| Property | Heat Resistance | Chemical Resistance |
|---|---|---|
| Definition | Ability to withstand elevated temperatures without deformation | Ability to resist degradation from chemical exposure |
| Temperature Range | Up to 80degC (176degF) continuous use | N/A |
| Common Chemicals Tested | N/A | Acids, bases, solvents (e.g., alcohols, ketones) |
| Performance | Low to moderate heat resistance; softens near 100degC | Resistant to dilute acids and bases; poor resistance to organic solvents |
| Applications | Electrical housings, packaging exposed to mild heat | Containers for mild chemicals, disposable labware |
| Limitations | Deforms above 100degC; not suitable for high-temp environments | Swells or dissolves in strong solvents; limited chemical durability |
Overview: Heat Resistance and Chemical Resistance in Polystyrene
Polystyrene exhibits moderate heat resistance, typically withstanding temperatures up to 70-90degC before deformation occurs, making it suitable for applications that involve low to moderate thermal exposure. Its chemical resistance varies significantly, showing good resistance to acids and alkalis but poor resistance to organic solvents like benzene and ketones, which can cause swelling or dissolution. Understanding the balance between polystyrene's thermal limits and its susceptibility to chemical attack is crucial for selecting appropriate uses in packaging, insulation, and consumer products.
Fundamental Properties of Polystyrene
Polystyrene exhibits moderate heat resistance with a glass transition temperature around 100degC, limiting its use in high-temperature applications. Chemically, it shows good resistance to aqueous solutions but is vulnerable to organic solvents like acetone and benzene, which cause swelling and degradation. These fundamental properties dictate polystyrene's suitability for disposable containers and insulation materials where chemical exposure is minimal and thermal stress is controlled.
Heat Resistance: Definition and Importance
Heat resistance in polystyrene refers to its ability to maintain structural integrity and performance when exposed to elevated temperatures, typically up to 70-90degC for general-purpose grades. This property is crucial for applications requiring thermal stability, such as food containers and insulation materials, to prevent deformation and degradation. Understanding heat resistance helps engineers select appropriate polystyrene grades for environments with moderate heat exposure, ensuring safety and durability.
Chemical Resistance: Definition and Significance
Chemical resistance in polystyrene refers to the material's ability to withstand degradation or damage when exposed to various chemicals, including acids, alcohols, and oils. This property is significant for applications where polystyrene containers or components encounter chemical substances, ensuring durability and maintaining structural integrity. Understanding chemical resistance aids in selecting appropriate polystyrene grades for packaging, laboratory ware, and automotive parts, minimizing material failure and contamination risks.
Comparing Heat and Chemical Resistance in Polystyrene
Polystyrene exhibits moderate heat resistance with a glass transition temperature around 100degC, limiting its use in high-temperature applications. Its chemical resistance is generally low, as it can be degraded by organic solvents like acetone and benzene. This combination necessitates careful consideration when selecting polystyrene for products exposed to heat or harsh chemicals.
Factors Affecting Heat Resistance of Polystyrene
Polystyrene's heat resistance is primarily influenced by its glass transition temperature, typically around 100degC, beyond which the material softens and loses structural integrity. Factors such as molecular weight, degree of crystallinity, and the presence of additives or fillers can enhance or diminish its thermal stability. While polystyrene offers moderate chemical resistance to acids and bases, its heat resistance is more critical in applications involving thermal exposure or hot liquids.
Factors Influencing Chemical Resistance of Polystyrene
Polystyrene exhibits moderate heat resistance, typically withstanding temperatures up to 100degC before deformation occurs, but its chemical resistance is substantially influenced by factors such as molecular weight, cross-linking density, and the presence of additives or plasticizers. Chemical resistance varies significantly depending on the type of solvent; for example, it resists weak acids and bases but degrades rapidly when exposed to organic solvents like benzene or acetone. Understanding these factors is crucial in applications where polystyrene is exposed to chemicals, ensuring durability and material integrity under specific environmental conditions.
Application Scenarios: When Heat Resistance Matters Most
Polystyrene's heat resistance is crucial in applications such as food packaging and microwave-safe containers, where exposure to elevated temperatures demands material stability to prevent deformation and maintain food safety. Chemical resistance, while generally limited in polystyrene, becomes secondary in scenarios dominated by thermal exposure rather than aggressive solvents or oils. In contrast, heat resistance is less critical in disposable or short-term use products where chemical interaction governs performance requirements.
Application Scenarios: Prioritizing Chemical Resistance
Polystyrene demonstrates moderate heat resistance, withstanding temperatures up to 80-100degC before deforming, but its chemical resistance is more critical in applications exposed to solvents and oils. In packaging and consumer products where contact with chemicals like alcohols, acids, or hydrocarbons occurs, prioritizing chemical resistance ensures material integrity and safety. Choosing polystyrene grades with enhanced chemical resistance extends product lifespan and maintains performance in harsh chemical environments.
Selecting Polystyrene for Your Application: Key Considerations
Polystyrene offers moderate heat resistance, with a maximum service temperature typically around 80-100degC, making it suitable for applications where high thermal stability is not critical. Chemical resistance varies; polystyrene resists acids and bases but is vulnerable to organic solvents like acetone and alcohol, which can degrade its structure. Selecting polystyrene requires balancing heat tolerance with exposure to chemicals, ensuring compatibility with the application's thermal demands and the chemical environment to maximize durability and performance.
Heat Resistance vs Chemical Resistance (in Polystyrene applications) Infographic
 materialdif.com
materialdif.com