Polyethylene Terephthalate (PET) offers superior clarity, strength, and chemical resistance compared to standard Polyethylene, making it ideal for packaging applications requiring durability and transparency. Unlike Polyethylene, which is more flexible and used primarily for films and bags, PET provides excellent gas and moisture barrier properties essential for food and beverage containers. The distinct molecular structures of PET and Polyethylene result in different thermal and mechanical performances, influencing their specific industrial uses.
Table of Comparison
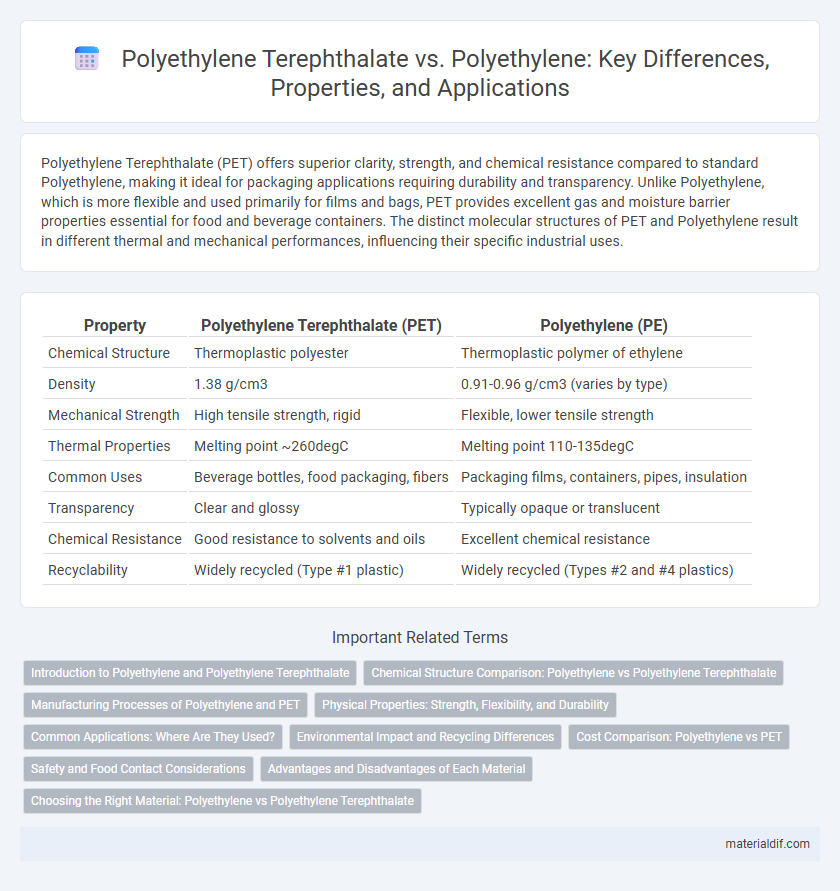
| Property | Polyethylene Terephthalate (PET) | Polyethylene (PE) |
|---|---|---|
| Chemical Structure | Thermoplastic polyester | Thermoplastic polymer of ethylene |
| Density | 1.38 g/cm3 | 0.91-0.96 g/cm3 (varies by type) |
| Mechanical Strength | High tensile strength, rigid | Flexible, lower tensile strength |
| Thermal Properties | Melting point ~260degC | Melting point 110-135degC |
| Common Uses | Beverage bottles, food packaging, fibers | Packaging films, containers, pipes, insulation |
| Transparency | Clear and glossy | Typically opaque or translucent |
| Chemical Resistance | Good resistance to solvents and oils | Excellent chemical resistance |
| Recyclability | Widely recycled (Type #1 plastic) | Widely recycled (Types #2 and #4 plastics) |
Introduction to Polyethylene and Polyethylene Terephthalate
Polyethylene is a versatile polymer widely used in packaging, containers, and plastic bags due to its durability and chemical resistance. Polyethylene Terephthalate (PET), a type of polyester, offers higher strength, thermal stability, and clarity, making it ideal for beverage bottles and food packaging. Both polymers are thermoplastics but differ significantly in molecular structure and applications, with polyethylene being a simple hydrocarbon chain and PET containing ester functional groups enhancing its mechanical and barrier properties.
Chemical Structure Comparison: Polyethylene vs Polyethylene Terephthalate
Polyethylene (PE) consists of long chains of ethylene monomers with a simple, non-polar hydrocarbon backbone, resulting in a flexible and chemically resistant structure. Polyethylene Terephthalate (PET) is a polyester formed through the condensation of terephthalic acid and ethylene glycol, featuring repeating ester functional groups that introduce polarity and rigidity. The chemical structure difference explains PET's superior thermal stability and mechanical strength compared to the more flexible and inert structure of PE.
Manufacturing Processes of Polyethylene and PET
Polyethylene (PE) is produced through the polymerization of ethylene monomers via processes such as high-pressure free-radical polymerization for low-density polyethylene (LDPE) and Ziegler-Natta or metallocene catalysis for high-density polyethylene (HDPE), resulting in varied structural properties. Polyethylene terephthalate (PET) synthesis involves a condensation polymerization reaction between terephthalic acid and ethylene glycol, followed by melt polymerization to achieve high molecular weight and crystallinity. These distinct manufacturing approaches define the differences in molecular architecture, thermal properties, and applications between PE and PET materials.
Physical Properties: Strength, Flexibility, and Durability
Polyethylene terephthalate (PET) exhibits higher tensile strength and rigidity compared to polyethylene (PE), making it ideal for applications requiring structural integrity. Polyethylene offers superior flexibility and impact resistance, which contributes to its widespread use in packaging films and containers. Both materials demonstrate excellent chemical resistance, but PET possesses enhanced durability under thermal stress, extending its functional lifespan in high-temperature environments.
Common Applications: Where Are They Used?
Polyethylene terephthalate (PET) is extensively used in beverage bottles, food packaging, and synthetic fibers due to its strong, lightweight, and transparent properties. Polyethylene (PE), including high-density (HDPE) and low-density (LDPE) variants, is commonly found in plastic bags, containers, pipes, and insulation materials because of its flexibility, chemical resistance, and durability. Both polymers serve crucial roles in packaging and manufacturing but are chosen based on specific application requirements such as strength, clarity, and environmental resistance.
Environmental Impact and Recycling Differences
Polyethylene terephthalate (PET) and polyethylene (PE) differ significantly in environmental impact and recycling processes. PET, commonly used in beverage bottles, is highly recyclable through established systems and can be processed into fibers, containers, and packaging materials, reducing landfill waste and lowering carbon emissions. In contrast, polyethylene, including low-density (LDPE) and high-density (HDPE) variants, is more chemically stable, making it resistant to biodegradation but versatile in recycling streams primarily for packaging, films, and containers, though its lower recycling rates contribute to greater environmental persistence.
Cost Comparison: Polyethylene vs PET
Polyethylene generally costs less per kilogram than Polyethylene Terephthalate (PET), making it the preferred choice for large-scale packaging and consumer goods production. PET's higher price is justified by its superior strength, clarity, and barrier properties, which are critical for applications like beverage bottles and food containers. Cost considerations often guide manufacturers in selecting polyethylene for lower-cost, high-volume applications, while reserving PET for products requiring enhanced durability and aesthetics.
Safety and Food Contact Considerations
Polyethylene Terephthalate (PET) is widely recognized for its excellent safety profile in food contact applications due to its strong resistance to chemical migration and ability to maintain product integrity at varying temperatures. In contrast, Polyethylene (PE), including both high-density (HDPE) and low-density (LDPE) variants, offers superior chemical inertness and flexibility but may have lower temperature resistance compared to PET. Both materials comply with FDA and EU food safety regulations, making them suitable for food packaging, though PET is often preferred for carbonated beverages and transparent containers, while PE is commonly used for milk jugs, squeezable bottles, and food wraps.
Advantages and Disadvantages of Each Material
Polyethylene Terephthalate (PET) offers superior strength, clarity, and chemical resistance, making it ideal for beverage containers and packaging, but it is less flexible and more expensive than Polyethylene (PE). Polyethylene, particularly Low-Density Polyethylene (LDPE) and High-Density Polyethylene (HDPE), provides excellent flexibility, impact resistance, and cost-effectiveness, though it lacks the thermal stability and rigidity of PET. PET's recyclability is well-established, contributing to sustainability in packaging, whereas PE recycling faces challenges due to diverse polymer densities and contamination issues.
Choosing the Right Material: Polyethylene vs Polyethylene Terephthalate
Polyethylene (PE) offers excellent chemical resistance and flexibility, making it ideal for packaging and plastic bags, while Polyethylene Terephthalate (PET) provides superior strength, clarity, and barrier properties suitable for beverage bottles and food containers. When choosing between PE and PET, consider application durability, transparency requirements, and recyclability, as PET typically offers higher tensile strength and better gas barrier performance. Cost factors and environmental impact, including the ease of recycling PET into new containers versus PE's widespread use in single-use plastics, also influence material selection.
Polyethylene Terephthalate vs Polyethylene Infographic
 materialdif.com
materialdif.com