Hard carbon exhibits a disordered, non-graphitizable structure with high thermal stability and excellent electrochemical performance, making it ideal for battery anodes. Soft carbon, or graphitizable carbon, transforms into crystalline graphite upon high-temperature treatment, offering better electrical conductivity but lower structural stability. Understanding the differences between hard and soft carbon aids in optimizing material selection for applications requiring specific mechanical and electrochemical properties.
Table of Comparison
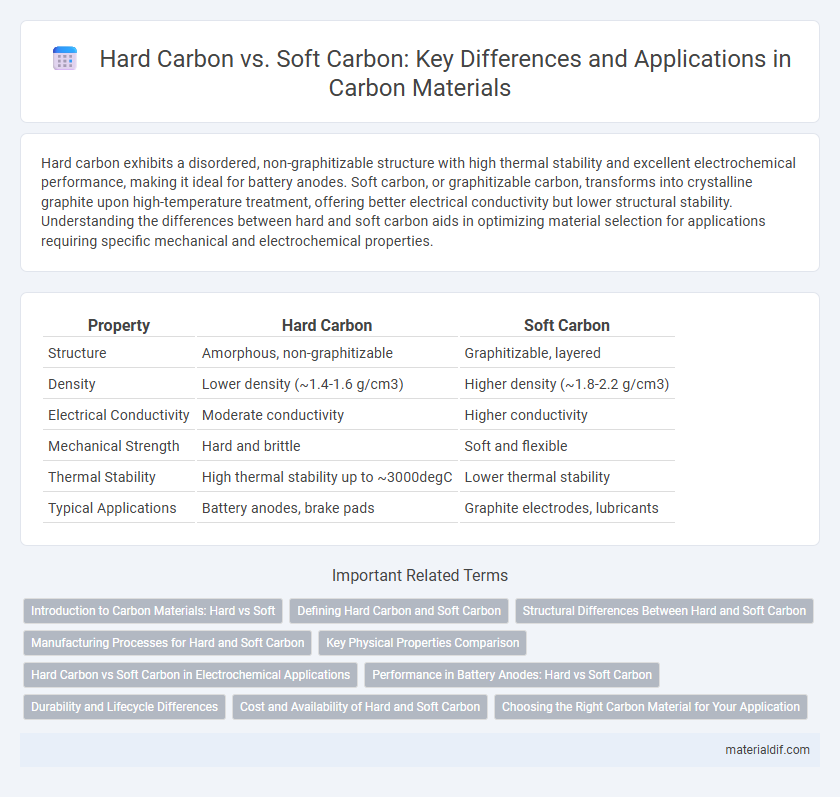
| Property | Hard Carbon | Soft Carbon |
|---|---|---|
| Structure | Amorphous, non-graphitizable | Graphitizable, layered |
| Density | Lower density (~1.4-1.6 g/cm3) | Higher density (~1.8-2.2 g/cm3) |
| Electrical Conductivity | Moderate conductivity | Higher conductivity |
| Mechanical Strength | Hard and brittle | Soft and flexible |
| Thermal Stability | High thermal stability up to ~3000degC | Lower thermal stability |
| Typical Applications | Battery anodes, brake pads | Graphite electrodes, lubricants |
Introduction to Carbon Materials: Hard vs Soft
Hard carbon, also known as non-graphitizable carbon, features a disordered structure with high porosity and low electrical conductivity, making it ideal for applications like battery anodes and filtration. Soft carbon, or graphitizable carbon, possesses an ordered layered structure that can convert into graphite upon heat treatment, offering superior electrical conductivity and mechanical strength for use in electrodes and conductive materials. Understanding the distinct microstructures and properties of hard and soft carbon materials is crucial for optimizing their performance in energy storage and composite technologies.
Defining Hard Carbon and Soft Carbon
Hard carbon, also known as non-graphitizable carbon, is characterized by its disordered, amorphous structure that resists graphitization even at high temperatures. Soft carbon, or graphitizable carbon, possesses a more ordered, graphitic microstructure that can transform into graphite when exposed to elevated heat. The fundamental distinction lies in their structural arrangement and thermal behavior, influencing their respective applications in energy storage and electrode materials.
Structural Differences Between Hard and Soft Carbon
Hard carbon features a highly disordered, non-graphitizable structure with randomly oriented graphene layers and abundant micropores, contributing to its rigid and dense nature. Soft carbon, or graphitizable carbon, consists of more ordered, stacked graphene layers that can rearrange into graphitic structures upon heat treatment, resulting in a more flexible and less dense material. These structural differences impact their electrochemical performance, with hard carbon favored in applications requiring stability and capacity in lithium-ion batteries, while soft carbon excels in conductivity and cyclability.
Manufacturing Processes for Hard and Soft Carbon
Hard carbon is typically produced through the pyrolysis of organic precursors such as resins or pitch at temperatures below 1200degC in an inert atmosphere, resulting in a non-graphitizable structure with high microporosity. Soft carbon, also known as graphitizable carbon, is manufactured by heat-treating materials like petroleum coke or coal tar pitch at higher temperatures above 2500degC, allowing the carbon atoms to rearrange into a more ordered, graphitic structure. The manufacturing process differences lead to distinct physical properties: hard carbon exhibits higher hardness and lower electrical conductivity, while soft carbon is more conductive and easier to graphitize.
Key Physical Properties Comparison
Hard carbon exhibits higher density and greater electrical conductivity compared to soft carbon, making it suitable for energy storage applications like batteries. Soft carbon typically features a more amorphous structure with lower density and enhanced flexibility, beneficial for structural and composite materials. The distinct microstructures result in significant differences in mechanical strength and thermal stability between hard and soft carbon.
Hard Carbon vs Soft Carbon in Electrochemical Applications
Hard carbon exhibits a disordered, non-graphitizable structure that delivers superior lithium-ion storage capacity and stable cycling performance in electrochemical applications, making it ideal for anode materials in sodium-ion batteries. Soft carbon, characterized by a graphitizable and more ordered structure, generally offers higher electrical conductivity but lower capacity retention under extensive cycling due to its tendency to form graphite layers. Electrochemical studies reveal that hard carbon's unique microstructure supports enhanced sodium ion intercalation and storage, whereas soft carbon favors fast charge-discharge rates but at the cost of long-term stability.
Performance in Battery Anodes: Hard vs Soft Carbon
Hard carbon exhibits superior electrochemical performance in battery anodes due to its higher capacity retention and better cycle stability compared to soft carbon. Its disordered structure allows for more efficient lithium-ion storage and slower degradation, resulting in enhanced battery lifespan and safety. Soft carbon, while offering faster ion diffusion rates, tends to suffer from lower capacity and reduced long-term durability in practical applications.
Durability and Lifecycle Differences
Hard carbon exhibits superior durability due to its densely packed graphite layers, resulting in longer lifecycle performance in applications such as battery anodes. Soft carbon, characterized by its less ordered structure, tends to experience faster capacity degradation and shorter operational lifespan. These structural differences directly influence the mechanical strength and cycle stability, making hard carbon the preferred choice for high-performance energy storage systems.
Cost and Availability of Hard and Soft Carbon
Hard carbon generally incurs higher production costs due to its complex manufacturing processes involving high-temperature pyrolysis and longer processing times, whereas soft carbon is more cost-effective, benefiting from simpler and faster production methods. Availability of soft carbon is typically greater because it can be derived from widely accessible raw materials like petroleum cokes, while hard carbon relies on specialized biomass precursors, limiting its large-scale supply. Market demand and specific application needs heavily influence the cost-effectiveness and procurement strategies for both hard and soft carbon types.
Choosing the Right Carbon Material for Your Application
Hard carbon offers higher structural stability and superior electrochemical performance, making it ideal for applications like high-capacity lithium-ion batteries and supercapacitors. Soft carbon provides better conductivity and easier graphitization, suitable for electrodes in energy storage devices requiring rapid charge-discharge cycles. Selecting between hard and soft carbon depends on specific requirements such as durability, conductivity, and application environment to optimize efficiency and longevity.
Hard Carbon vs Soft Carbon Infographic
 materialdif.com
materialdif.com