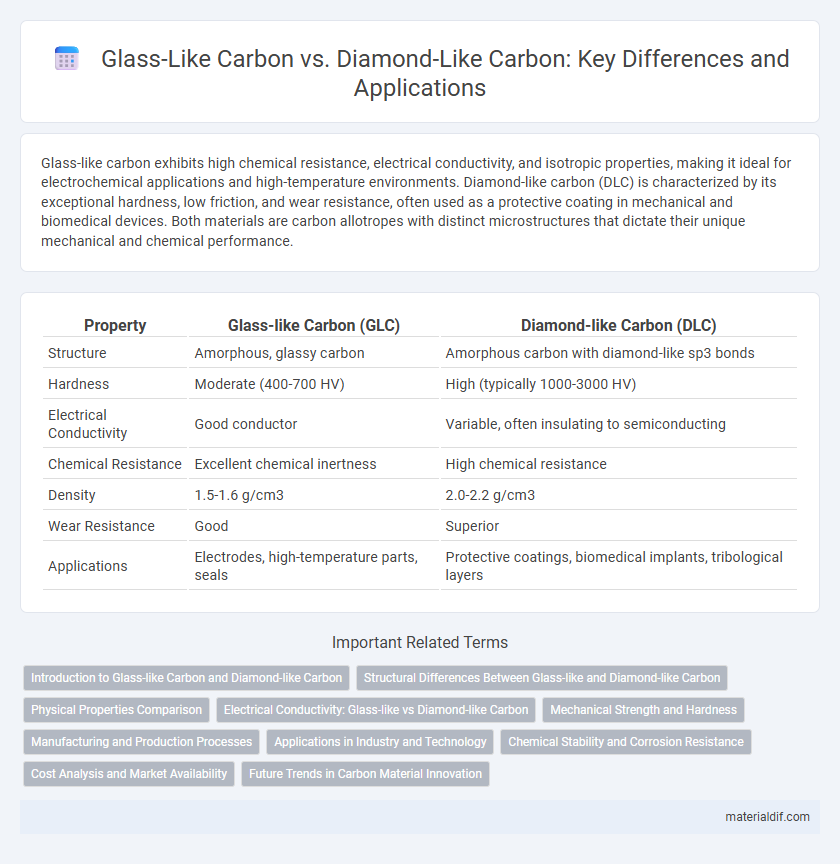
Glass-like carbon exhibits high chemical resistance, electrical conductivity, and isotropic properties, making it ideal for electrochemical applications and high-temperature environments. Diamond-like carbon (DLC) is characterized by its exceptional hardness, low friction, and wear resistance, often used as a protective coating in mechanical and biomedical devices. Both materials are carbon allotropes with distinct microstructures that dictate their unique mechanical and chemical performance.
Table of Comparison
| Property | Glass-like Carbon (GLC) | Diamond-like Carbon (DLC) |
|---|---|---|
| Structure | Amorphous, glassy carbon | Amorphous carbon with diamond-like sp3 bonds |
| Hardness | Moderate (400-700 HV) | High (typically 1000-3000 HV) |
| Electrical Conductivity | Good conductor | Variable, often insulating to semiconducting |
| Chemical Resistance | Excellent chemical inertness | High chemical resistance |
| Density | 1.5-1.6 g/cm3 | 2.0-2.2 g/cm3 |
| Wear Resistance | Good | Superior |
| Applications | Electrodes, high-temperature parts, seals | Protective coatings, biomedical implants, tribological layers |
Introduction to Glass-like Carbon and Diamond-like Carbon
Glass-like carbon exhibits a unique combination of glassy and graphite-like properties, featuring high chemical resistance, low density, and exceptional thermal stability. Diamond-like carbon (DLC) consists of amorphous carbon with significant sp3 bonding, offering high hardness, low friction, and excellent wear resistance similar to diamond. Both materials serve critical roles in advanced coatings, electronics, and medical implants due to their distinct structural and mechanical characteristics.
Structural Differences Between Glass-like and Diamond-like Carbon
Glass-like carbon exhibits a predominantly sp2 hybridized graphitic structure with a disordered, amorphous arrangement, resulting in high electrical conductivity and chemical inertness. Diamond-like carbon (DLC), by contrast, contains a significant sp3 hybridization resembling diamond's tetrahedral lattice, which imparts exceptional hardness and low friction properties. The structural disparity between the planar, sheet-like layers of glass-like carbon and the dense, three-dimensional network of DLC defines their distinct mechanical and electrical characteristics.
Physical Properties Comparison
Glass-like carbon exhibits high thermal stability, low density, and excellent chemical resistance, making it suitable for high-temperature applications, while diamond-like carbon (DLC) is characterized by exceptional hardness, low friction coefficient, and high wear resistance. Both materials have unique physical properties: glass-like carbon combines isotropic electrical conductivity with impermeability to gases, whereas DLC offers superior optical transparency and biocompatibility. The structural differences, with glass-like carbon having a graphitic microstructure and DLC featuring a mixture of sp2 and sp3 bonds, directly influence their mechanical strength and surface properties.
Electrical Conductivity: Glass-like vs Diamond-like Carbon
Glass-like carbon exhibits high electrical conductivity due to its sp2-bonded carbon atoms forming a continuous conjugated network, making it suitable for electrodes and electronic applications. Diamond-like carbon (DLC), primarily composed of sp3-bonded carbon atoms, has significantly lower electrical conductivity, functioning more as an electrical insulator. The distinct bonding structures directly influence their electron mobility and charge transport properties, with glass-like carbon enabling efficient conduction compared to the resistive nature of diamond-like carbon.
Mechanical Strength and Hardness
Glass-like carbon exhibits high mechanical strength with excellent resistance to compression and chemical corrosion, characterized by a unique combination of hardness and low density. Diamond-like carbon (DLC) films provide superior hardness, often approaching that of natural diamond, with exceptional wear resistance and low friction properties. In comparison, DLC offers higher hardness and greater surface durability, while glass-like carbon excels in structural strength and thermal stability under mechanical stress.
Manufacturing and Production Processes
Glass-like carbon is produced through the pyrolysis of polymers in an inert atmosphere, resulting in a non-graphitizing carbon material with a highly porous and isotropic structure. Diamond-like carbon (DLC) coatings are typically manufactured using physical vapor deposition (PVD) or chemical vapor deposition (CVD) techniques, which enable the formation of thin, hard, and amorphous carbon films with diamond-like properties. Both production processes differ significantly, with glass-like carbon emphasizing bulk polymer transformations and DLC focusing on surface engineering via advanced deposition methods.
Applications in Industry and Technology
Glass-like carbon exhibits exceptional chemical stability and high thermal conductivity, making it ideal for use in electrodes, high-temperature furnace components, and fuel cell technologies. Diamond-like carbon coatings provide superior hardness, low friction, and wear resistance, finding applications in automotive engine parts, cutting tools, and medical implants. Both materials play crucial roles in advancing electronics, energy storage devices, and surface engineering due to their unique carbon allotrope properties.
Chemical Stability and Corrosion Resistance
Glass-like carbon exhibits exceptional chemical stability due to its predominantly sp2-bonded carbon structure, rendering it highly resistant to acids, alkalis, and oxidative environments. Diamond-like carbon (DLC), with a mixture of sp2 and sp3 bonds, offers excellent corrosion resistance, particularly in abrasive and high-wear applications, thanks to its dense, amorphous carbon matrix. Both materials provide superior corrosion resistance compared to metals, but glass-like carbon excels in harsh chemical environments while DLC is favored for protective coatings in mechanical systems.
Cost Analysis and Market Availability
Glass-like carbon offers lower production costs due to its simpler manufacturing process compared to diamond-like carbon, which requires more complex chemical vapor deposition techniques. Market availability of glass-like carbon is relatively higher, with widespread applications in electrodes and coatings, whereas diamond-like carbon remains niche, primarily used in high-performance wear-resistant coatings. Pricing disparities impact industrial adoption, with glass-like carbon favored in cost-sensitive sectors and diamond-like carbon preferred for premium applications demanding superior hardness and chemical stability.
Future Trends in Carbon Material Innovation
Glass-like carbon exhibits exceptional chemical stability and electrical conductivity, making it ideal for next-generation energy storage and sensor technologies. Diamond-like carbon offers superior hardness and low friction, driving advancements in wear-resistant coatings and biomedical implants. Emerging hybrid carbon materials that combine these properties are poised to revolutionize flexible electronics and sustainable manufacturing.
Glass-like carbon vs Diamond-like carbon Infographic
 materialdif.com
materialdif.com