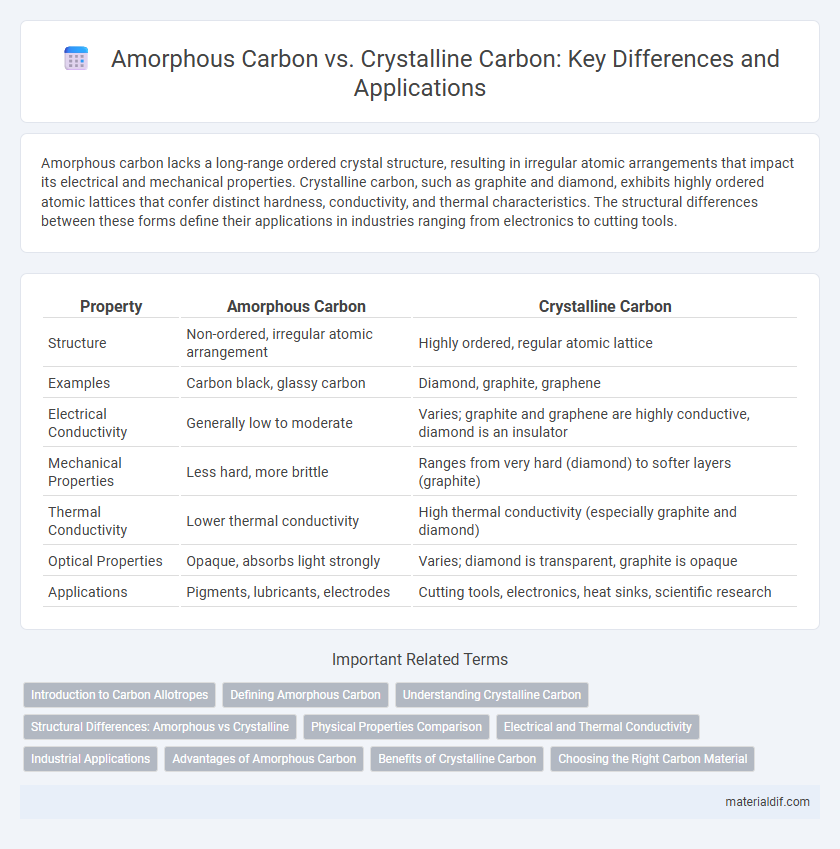
Amorphous carbon lacks a long-range ordered crystal structure, resulting in irregular atomic arrangements that impact its electrical and mechanical properties. Crystalline carbon, such as graphite and diamond, exhibits highly ordered atomic lattices that confer distinct hardness, conductivity, and thermal characteristics. The structural differences between these forms define their applications in industries ranging from electronics to cutting tools.
Table of Comparison
| Property | Amorphous Carbon | Crystalline Carbon |
|---|---|---|
| Structure | Non-ordered, irregular atomic arrangement | Highly ordered, regular atomic lattice |
| Examples | Carbon black, glassy carbon | Diamond, graphite, graphene |
| Electrical Conductivity | Generally low to moderate | Varies; graphite and graphene are highly conductive, diamond is an insulator |
| Mechanical Properties | Less hard, more brittle | Ranges from very hard (diamond) to softer layers (graphite) |
| Thermal Conductivity | Lower thermal conductivity | High thermal conductivity (especially graphite and diamond) |
| Optical Properties | Opaque, absorbs light strongly | Varies; diamond is transparent, graphite is opaque |
| Applications | Pigments, lubricants, electrodes | Cutting tools, electronics, heat sinks, scientific research |
Introduction to Carbon Allotropes
Carbon allotropes exhibit diverse structural forms primarily categorized into amorphous and crystalline types, each with distinct physical and chemical properties. Amorphous carbon lacks a long-range ordered crystal structure, resulting in materials like charcoal and carbon black that are highly porous and exhibit variable hardness. Crystalline carbon allotropes, such as diamond and graphite, display highly ordered atomic arrangements, contributing to their exceptional hardness and electrical conductivity, respectively.
Defining Amorphous Carbon
Amorphous carbon is a non-crystalline form of carbon lacking a long-range ordered atomic structure, distinguishing it from crystalline carbon allotropes like graphite and diamond. It consists of a random arrangement of carbon atoms with varying hybridization states, primarily sp2 and sp3 bonds, resulting in unique electrical, mechanical, and optical properties. Applications of amorphous carbon include hard coatings, thin films, and electronic devices where high hardness and chemical inertness are required.
Understanding Crystalline Carbon
Crystalline carbon, characterized by a highly ordered atomic structure, includes forms such as diamond and graphite, where carbon atoms are arranged in repeating patterns that give rise to distinct physical properties. The atomic lattice in crystalline carbon results in superior hardness, thermal conductivity, and electrical properties compared to amorphous carbon. Understanding the difference in bonding and structural organization is essential for applications in electronics, abrasives, and nanotechnology.
Structural Differences: Amorphous vs Crystalline
Amorphous carbon lacks a long-range ordered atomic structure, characterized by random, irregular bonding patterns, whereas crystalline carbon exhibits a highly ordered lattice arrangement such as graphite or diamond. The sp2 and sp3 hybridization ratios vary significantly between these forms, with amorphous carbon containing a mix of bonding types, leading to distinct electrical and mechanical properties compared to the uniform hybridization found in crystalline carbon. Structural differences influence the material's conductivity, hardness, and chemical reactivity, making crystalline carbon materials ideal for applications requiring stability and strength, while amorphous carbon suits flexible, conductive coatings.
Physical Properties Comparison
Amorphous carbon lacks a long-range ordered atomic structure, resulting in lower density and electrical conductivity compared to crystalline carbon forms such as graphite and diamond, which exhibit highly organized lattices. Crystalline carbon variants often possess superior hardness and thermal conductivity, with diamond being the hardest known natural material and graphite displaying excellent lubricity and anisotropic conductivity. The disparity in physical properties between amorphous and crystalline carbon is primarily due to differences in atomic bonding and structural arrangement, impacting their applications in electronics, coatings, and composite materials.
Electrical and Thermal Conductivity
Amorphous carbon exhibits lower electrical and thermal conductivity compared to crystalline carbon due to its disordered atomic structure, which limits electron and phonon transport. Crystalline carbon forms such as graphite and diamond possess highly ordered lattices, enabling efficient electron mobility and heat conduction; graphite shows excellent electrical conductivity along its planes, while diamond demonstrates superior thermal conductivity. The variance in conductivity between amorphous and crystalline carbon significantly impacts applications in electronics and thermal management.
Industrial Applications
Amorphous carbon, characterized by its non-crystalline structure and high surface area, is widely utilized in industrial applications such as ink production, battery electrodes, and filtration systems due to its excellent electrical conductivity and adsorption properties. Crystalline carbon, including graphite and diamond, offers superior hardness and thermal conductivity, making it essential for cutting tools, lubricants, and heat sinks in high-performance industrial settings. The selection between amorphous and crystalline carbon depends on specific application requirements like electrical conductivity, mechanical strength, and thermal stability.
Advantages of Amorphous Carbon
Amorphous carbon offers significant advantages over crystalline carbon due to its disordered atomic structure, resulting in superior hardness and enhanced chemical stability. Its isotropic properties make it highly resistant to wear and corrosion, ideal for protective coatings in harsh environments. Additionally, amorphous carbon exhibits excellent electrical conductivity and tunable optical characteristics, expanding its applications in electronics and photonics.
Benefits of Crystalline Carbon
Crystalline carbon, exemplified by diamond and graphite, exhibits superior structural stability and exceptional hardness, making it ideal for industrial applications requiring durability. Its well-ordered atomic arrangement allows for high thermal conductivity and electrical properties, beneficial in electronics and heat management systems. Crystalline carbon's consistent lattice structure enhances material performance and reliability in scientific and technological fields.
Choosing the Right Carbon Material
Amorphous carbon exhibits a disordered atomic structure, resulting in high surface area and excellent electrical conductivity, making it ideal for applications like electrodes and supercapacitors. Crystalline carbon, including graphite and diamond, offers superior mechanical strength and thermal conductivity, preferred in cutting tools and heat sinks. Selecting the right carbon material depends on balancing properties such as conductivity, hardness, and surface characteristics to meet specific industrial or technological requirements.
Amorphous Carbon vs Crystalline Carbon Infographic
 materialdif.com
materialdif.com