Activated carbon and graphene are both carbon-based materials with unique properties suited for different applications. Activated carbon features a highly porous structure ideal for adsorption and filtration, while graphene consists of a single layer of carbon atoms offering exceptional electrical conductivity and mechanical strength. Choosing between activated carbon and graphene depends on specific needs such as surface area for purification or conductivity for electronics.
Table of Comparison
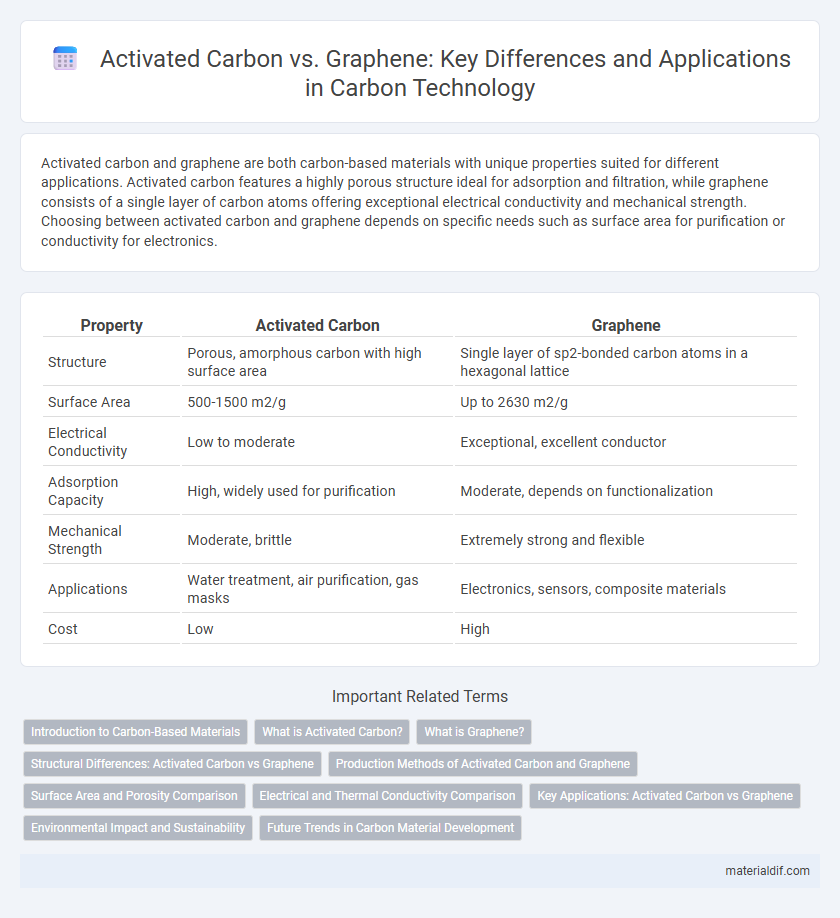
| Property | Activated Carbon | Graphene |
|---|---|---|
| Structure | Porous, amorphous carbon with high surface area | Single layer of sp2-bonded carbon atoms in a hexagonal lattice |
| Surface Area | 500-1500 m2/g | Up to 2630 m2/g |
| Electrical Conductivity | Low to moderate | Exceptional, excellent conductor |
| Adsorption Capacity | High, widely used for purification | Moderate, depends on functionalization |
| Mechanical Strength | Moderate, brittle | Extremely strong and flexible |
| Applications | Water treatment, air purification, gas masks | Electronics, sensors, composite materials |
| Cost | Low | High |
Introduction to Carbon-Based Materials
Activated carbon and graphene are two prominent carbon-based materials distinguished by their structure and applications. Activated carbon features a highly porous structure that provides exceptional adsorption capacity, widely utilized in filtration and purification processes. Graphene consists of a single layer of carbon atoms arranged in a hexagonal lattice, offering extraordinary electrical conductivity, mechanical strength, and thermal properties for advanced electronic and composite materials.
What is Activated Carbon?
Activated carbon is a highly porous form of carbon processed to have a large surface area, making it effective for adsorption in air and water purification. It is produced by heating carbon-rich materials like coconut shells or coal at high temperatures in the presence of gases, creating a network of tiny pores. This unique structure enables activated carbon to trap contaminants, odors, and chemicals, distinguishing it from materials like graphene with different properties and applications.
What is Graphene?
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, known for its exceptional electrical conductivity, mechanical strength, and thermal properties. Unlike activated carbon, which has a porous, amorphous structure mainly used for adsorption and filtration, graphene's atom-thin, crystalline structure enables advanced applications in electronics, energy storage, and sensors. Its unique combination of conductivity and flexibility distinguishes graphene as a revolutionary material beyond traditional carbon forms.
Structural Differences: Activated Carbon vs Graphene
Activated carbon consists of a highly porous, amorphous structure with a large surface area optimized for adsorption, formed by the random arrangement of carbon atoms creating micropores and mesopores. Graphene features a two-dimensional, single layer of sp2-bonded carbon atoms arranged in a hexagonal lattice, enabling exceptional electrical conductivity and mechanical strength. The key structural difference lies in activated carbon's disordered, porous morphology versus graphene's ordered, crystalline atomic arrangement.
Production Methods of Activated Carbon and Graphene
Activated carbon is primarily produced through physical activation, involving carbonization of raw materials like coconut shells or coal followed by oxidation at high temperatures, or chemical activation using agents such as phosphoric acid or potassium hydroxide to enhance porosity. Graphene production methods include mechanical exfoliation, chemical vapor deposition (CVD) on metal substrates, and chemical reduction of graphene oxide, each offering different scalability and quality. While activated carbon production emphasizes surface area and porosity for adsorption applications, graphene synthesis focuses on achieving single-layer sheets with exceptional electrical and mechanical properties.
Surface Area and Porosity Comparison
Activated carbon typically exhibits a surface area ranging from 500 to 1500 m2/g, characterized by a highly porous structure with micropores and mesopores that enhance adsorption capabilities. Graphene, in contrast, boasts an exceptional theoretical surface area of approximately 2630 m2/g, primarily as a two-dimensional material with minimal intrinsic porosity but significant potential for surface modification. The porosity of activated carbon enables superior adsorption for bulkier molecules, while graphene's high surface area and tunable functionalization offer advantages in electronic applications and selective adsorption processes.
Electrical and Thermal Conductivity Comparison
Activated carbon exhibits moderate electrical conductivity due to its porous amorphous structure, making it suitable for applications like supercapacitors but limited in high-performance electronic devices. Graphene, a single layer of sp2-bonded carbon atoms arranged in a hexagonal lattice, demonstrates exceptional electrical conductivity with electron mobility reaching up to 200,000 cm2/V*s and superior thermal conductivity exceeding 5,000 W/m*K, outperforming activated carbon significantly. This remarkable conductivity of graphene facilitates faster electron transport and efficient heat dissipation, essential for advanced electronics and thermal management systems.
Key Applications: Activated Carbon vs Graphene
Activated carbon excels in water purification, air filtration, and industrial solvent recovery due to its high surface area and porosity, making it ideal for adsorption processes. Graphene, with its exceptional electrical conductivity and mechanical strength, is primarily used in electronics, energy storage devices such as supercapacitors, and advanced composite materials. While activated carbon targets environmental remediation and chemical processing, graphene's key applications focus on next-generation electronics and high-performance materials.
Environmental Impact and Sustainability
Activated carbon, derived mainly from biomass or coal, offers high adsorption capacity for pollutants, making it effective in water and air purification while being biodegradable and renewable when sourced sustainably. Graphene, a single layer of carbon atoms with exceptional strength and conductivity, involves energy-intensive production methods, often relying on hazardous chemicals, which raises concerns about its environmental footprint and scalability. Sustainable development favors activated carbon for widespread environmental remediation, whereas advancements in greener graphene synthesis are crucial to reduce its ecological impact.
Future Trends in Carbon Material Development
Future trends in carbon material development emphasize the integration of activated carbon's high surface area with graphene's exceptional electrical conductivity to enhance energy storage and filtration technologies. Research is advancing toward hybrid composites that leverage activated carbon's porous structure alongside graphene's mechanical strength for next-generation supercapacitors and water purification systems. Emerging applications target sustainable energy solutions, aiming to maximize carbon materials' efficiency and environmental impact reduction.
Activated Carbon vs Graphene Infographic
 materialdif.com
materialdif.com