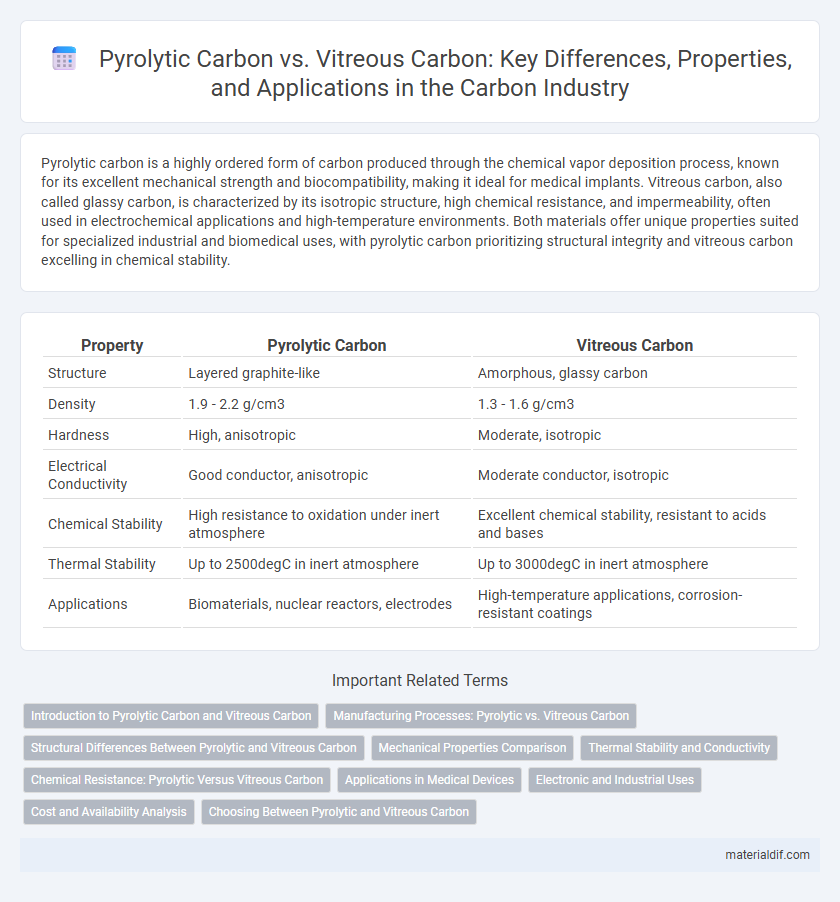
Pyrolytic carbon is a highly ordered form of carbon produced through the chemical vapor deposition process, known for its excellent mechanical strength and biocompatibility, making it ideal for medical implants. Vitreous carbon, also called glassy carbon, is characterized by its isotropic structure, high chemical resistance, and impermeability, often used in electrochemical applications and high-temperature environments. Both materials offer unique properties suited for specialized industrial and biomedical uses, with pyrolytic carbon prioritizing structural integrity and vitreous carbon excelling in chemical stability.
Table of Comparison
| Property | Pyrolytic Carbon | Vitreous Carbon |
|---|---|---|
| Structure | Layered graphite-like | Amorphous, glassy carbon |
| Density | 1.9 - 2.2 g/cm3 | 1.3 - 1.6 g/cm3 |
| Hardness | High, anisotropic | Moderate, isotropic |
| Electrical Conductivity | Good conductor, anisotropic | Moderate conductor, isotropic |
| Chemical Stability | High resistance to oxidation under inert atmosphere | Excellent chemical stability, resistant to acids and bases |
| Thermal Stability | Up to 2500degC in inert atmosphere | Up to 3000degC in inert atmosphere |
| Applications | Biomaterials, nuclear reactors, electrodes | High-temperature applications, corrosion-resistant coatings |
Introduction to Pyrolytic Carbon and Vitreous Carbon
Pyrolytic carbon is a high-purity form of carbon produced by the thermal decomposition of hydrocarbon gases in a controlled environment, resulting in a layered, graphitic microstructure with excellent mechanical strength and biocompatibility. Vitreous carbon, also known as glassy carbon, is a non-graphitizing carbon material characterized by its isotropic structure, high chemical resistance, and low density, often used in electrochemical applications. Both materials exhibit unique physical and chemical properties tailored for specialized industrial, medical, and scientific uses.
Manufacturing Processes: Pyrolytic vs. Vitreous Carbon
Pyrolytic carbon is manufactured through chemical vapor deposition, where hydrocarbon gases decompose at high temperatures to form layered carbon with anisotropic properties. Vitreous carbon is produced by the carbonization of polymer precursors, involving high-temperature pyrolysis that results in a non-graphitizing, isotropic, glass-like carbon structure. The distinct manufacturing processes dictate their microstructure and mechanical behaviors, with pyrolytic carbon exhibiting directional strength and vitreous carbon demonstrating exceptional hardness and chemical resistance.
Structural Differences Between Pyrolytic and Vitreous Carbon
Pyrolytic carbon exhibits a highly ordered, layered graphitic structure formed through the chemical vapor deposition process, resulting in anisotropic properties. Vitreous carbon, on the other hand, possesses an isotropic, non-graphitizing amorphous structure characterized by a three-dimensional network of disordered carbon atoms. These structural differences contribute to pyrolytic carbon's superior electrical conductivity and thermal stability compared to the chemically inert and corrosion-resistant nature of vitreous carbon.
Mechanical Properties Comparison
Pyrolytic carbon exhibits high strength and excellent resistance to wear and fatigue due to its layered graphite structure, making it ideal for applications requiring durability under cyclic stress. Vitreous carbon combines the hardness and brittle nature of glass with the chemical resistance and low density of carbon, providing superior compressive strength but lower tensile strength compared to pyrolytic carbon. The mechanical properties of pyrolytic carbon favor impact resistance and flexibility, while vitreous carbon excels in hardness and thermal stability under compression.
Thermal Stability and Conductivity
Pyrolytic carbon exhibits exceptional thermal stability up to 2000degC and high anisotropic thermal conductivity, making it ideal for high-temperature applications requiring efficient heat dissipation. Vitreous carbon, while also stable at elevated temperatures (up to 3000degC), has isotropic thermal conductivity that is generally lower than that of pyrolytic carbon. Both materials offer unique advantages in thermal management, but pyrolytic carbon's directional conductivity and moderate thermal stability favor its use in advanced electronic and aerospace components.
Chemical Resistance: Pyrolytic Versus Vitreous Carbon
Pyrolytic carbon exhibits superior chemical resistance due to its highly ordered graphitic layers, making it more resistant to acids, alkalis, and oxidizing environments compared to vitreous carbon. Vitreous carbon, though chemically inert and able to withstand high temperatures, shows lower resistance to strong oxidative conditions and certain aggressive chemicals. The anisotropic nature of pyrolytic carbon's microstructure enhances its durability and stability, especially in harsh chemical applications.
Applications in Medical Devices
Pyrolytic carbon is extensively used in heart valve prostheses and orthopedic implants due to its exceptional biocompatibility, wear resistance, and thromboresistance properties, ensuring long-term durability and minimal immune response. Vitreous carbon, characterized by its high compressive strength and chemical inertness, is preferred in electrode fabrication and implantable sensors, offering stability in corrosive bodily environments. Both materials play critical roles in medical devices, with pyrolytic carbon dominating applications requiring dynamic mechanical performance and vitreous carbon excelling in static, chemically demanding settings.
Electronic and Industrial Uses
Pyrolytic carbon exhibits high electrical conductivity and excellent thermal stability, making it ideal for electronic components such as microelectrodes, sensors, and capacitors. Vitreous carbon offers superior chemical resistance and low permeability alongside good electrical conductivity, which benefits industrial applications like corrosion-resistant coatings, high-temperature crucibles, and electrode materials in electrochemical processes. Both forms cater to specialized electronic and industrial needs due to their unique structural and conductive properties.
Cost and Availability Analysis
Pyrolytic carbon is generally more expensive due to its complex production process involving chemical vapor deposition, making it less widely available compared to vitreous carbon. Vitreous carbon offers a cost-effective alternative with broader availability, produced through the high-temperature carbonization of polymer precursors. Both materials serve specific applications, but vitreous carbon's lower cost and easier accessibility make it preferable for large-scale industrial uses.
Choosing Between Pyrolytic and Vitreous Carbon
Choosing between pyrolytic carbon and vitreous carbon depends on the specific application requirements such as mechanical strength, thermal stability, and chemical resistance. Pyrolytic carbon offers excellent structural integrity and is ideal for biomedical implants and high-temperature environments due to its layered structure and anisotropic properties. Vitreous carbon, characterized by its isotropic nature and high chemical inertness, suits applications requiring corrosion resistance and electrical conductivity, such as electrodes and high-performance chemical reactors.
Pyrolytic carbon vs Vitreous carbon Infographic
 materialdif.com
materialdif.com