Diamond and lonsdaleite are both carbon allotropes with distinct crystal structures that influence their hardness and industrial applications. Lonsdaleite, often called hexagonal diamond, exhibits a hexagonal lattice structure, making it theoretically harder than the cubic lattice diamond under specific conditions. The unique atomic arrangement of lonsdaleite provides enhanced resistance to deformation, positioning it as a promising material for cutting tools and high-pressure experiments.
Table of Comparison
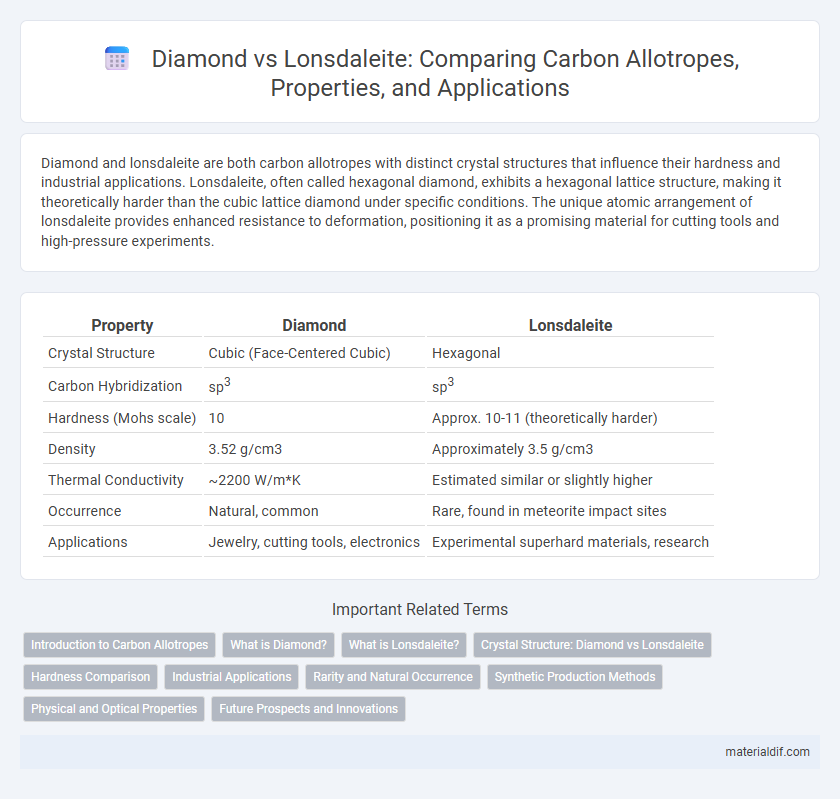
| Property | Diamond | Lonsdaleite |
|---|---|---|
| Crystal Structure | Cubic (Face-Centered Cubic) | Hexagonal |
| Carbon Hybridization | sp3 | sp3 |
| Hardness (Mohs scale) | 10 | Approx. 10-11 (theoretically harder) |
| Density | 3.52 g/cm3 | Approximately 3.5 g/cm3 |
| Thermal Conductivity | ~2200 W/m*K | Estimated similar or slightly higher |
| Occurrence | Natural, common | Rare, found in meteorite impact sites |
| Applications | Jewelry, cutting tools, electronics | Experimental superhard materials, research |
Introduction to Carbon Allotropes
Carbon allotropes exhibit distinct crystal structures that define their unique properties, with diamond and lonsdaleite as notable examples. Diamond features a cubic crystal lattice formed by strong sp3 carbon-carbon bonds, resulting in exceptional hardness and thermal conductivity. Lonsdaleite, also known as hexagonal diamond, possesses a hexagonal lattice that imparts comparable hardness but slightly different mechanical characteristics, making it a subject of interest in materials science.
What is Diamond?
Diamond is a crystalline form of carbon characterized by a cubic crystal structure, renowned for its exceptional hardness and high refractive index. Composed of carbon atoms tetrahedrally bonded in a rigid lattice, diamonds exhibit superior thermal conductivity and optical transparency. These properties distinguish diamond from lonsdaleite, which has a hexagonal crystal structure and slightly different physical characteristics.
What is Lonsdaleite?
Lonsdaleite is a rare hexagonal allotrope of carbon, structurally distinct from the cubic lattice of diamond but similarly composed of carbon atoms. Formed naturally during meteorite impacts when graphite undergoes rapid high-pressure and high-temperature transformation, it exhibits unique hardness properties potentially surpassing diamond. Its hexagonal crystal structure alters physical characteristics, making lonsdaleite a subject of interest for advanced material science and industrial applications.
Crystal Structure: Diamond vs Lonsdaleite
Diamond exhibits a face-centered cubic (FCC) crystal structure with each carbon atom tetrahedrally bonded to four others, resulting in exceptional hardness and thermal conductivity. Lonsdaleite, also known as hexagonal diamond, features a hexagonal crystal lattice, where carbon atoms form a hexagonal arrangement akin to graphite but with sp3 hybridization. The differing crystal structures influence their mechanical properties, with Lonsdaleite theoretically surpassing diamond's hardness due to its hexagonal symmetry and bond orientation.
Hardness Comparison
Diamond, composed of carbon atoms arranged in a cubic crystal structure, exhibits a hardness rating of 10 on the Mohs scale, making it the hardest known natural material. Lonsdaleite, also known as hexagonal diamond, possesses a hexagonal crystal lattice that theoretical models and experimental studies suggest may exceed diamond's hardness by up to 58%, potentially reaching values above 70 GPa in Vickers hardness. This exceptional hardness of lonsdaleite is attributed to its unique atomic arrangement, which provides greater resistance to deformation under high-pressure conditions.
Industrial Applications
Diamond and lonsdaleite, both allotropes of carbon, exhibit exceptional hardness with diamond having a Mohs hardness of 10 and lonsdaleite potentially surpassing this with theoretical values around 10.5. Industrial applications leverage diamond's unparalleled thermal conductivity and abrasion resistance in cutting, grinding, and drilling tools, while lonsdaleite's unique crystallographic structure suggests potential for superior performance in extreme wear conditions and high-pressure environments. Research into lonsdaleite is expanding its use in advanced manufacturing sectors, particularly where materials with exceptional strength-to-weight ratios are critical.
Rarity and Natural Occurrence
Lonsdaleite is an extremely rare carbon allotrope found naturally in meteorite impact sites, contrasting with diamonds which are abundant and formed deep within Earth's mantle. While diamonds have widespread geological distribution and commercial availability, lonsdaleite's scarcity is attributed to its limited formation conditions and transient existence. This rarity makes lonsdaleite a subject of scientific interest but restricts its natural availability compared to the commonality of diamonds.
Synthetic Production Methods
Synthetic production of diamond primarily employs high-pressure high-temperature (HPHT) and chemical vapor deposition (CVD) methods, replicating natural conditions to grow high-purity crystals. Lonsdaleite synthesis remains challenging due to its hexagonal lattice structure, with recent advances leveraging shockwave compression of graphite and chemical vapor deposition under specific conditions to stabilize this rare carbon allotrope. Innovations in plasma-enhanced CVD and catalyst-assisted HPHT have improved the quality and scalability of synthetic lonsdaleite, opening pathways for its potential applications in cutting and abrasive technologies.
Physical and Optical Properties
Diamond exhibits exceptional hardness with a Mohs scale rating of 10 and a refractive index of 2.42, contributing to its brilliant luster and superior light dispersion. Lonsdaleite, also known as hexagonal diamond, possesses a slightly lower hardness estimated around 8.5 to 9.5 and demonstrates a refractive index close to 2.40, with subtle differences in optical anisotropy due to its hexagonal crystal structure. The distinct lattice arrangement in lonsdaleite affects its mechanical strength and optical properties compared to the cubic symmetry of traditional diamonds.
Future Prospects and Innovations
Diamond and lonsdaleite, both carbon allotropes with distinct crystal structures, exhibit unique properties that drive future material innovations. Lonsdaleite's hexagonal lattice offers potential for greater hardness and thermal conductivity than traditional cubic diamond, promising advancements in cutting tools and electronics. Emerging research explores synthetic production methods and nanoscale applications, positioning lonsdaleite as a game-changer in superhard materials and quantum computing technologies.
Diamond vs Lonsdaleite Infographic
 materialdif.com
materialdif.com