Synthetic carbon offers enhanced purity and uniformity compared to natural carbon, making it ideal for high-performance applications such as electronics and aerospace. Natural carbon, derived from coal or biomass, contains impurities and variable structures that can limit its effectiveness in precision uses. Advances in synthetic carbon production enable tailored properties that improve strength, conductivity, and durability beyond those of natural carbon materials.
Table of Comparison
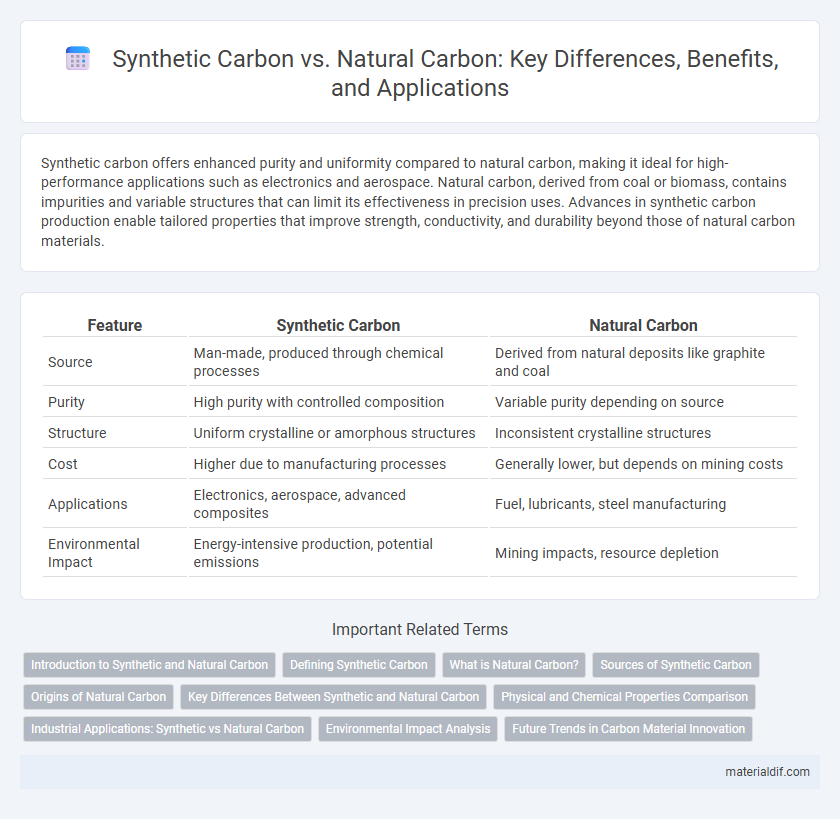
| Feature | Synthetic Carbon | Natural Carbon |
|---|---|---|
| Source | Man-made, produced through chemical processes | Derived from natural deposits like graphite and coal |
| Purity | High purity with controlled composition | Variable purity depending on source |
| Structure | Uniform crystalline or amorphous structures | Inconsistent crystalline structures |
| Cost | Higher due to manufacturing processes | Generally lower, but depends on mining costs |
| Applications | Electronics, aerospace, advanced composites | Fuel, lubricants, steel manufacturing |
| Environmental Impact | Energy-intensive production, potential emissions | Mining impacts, resource depletion |
Introduction to Synthetic and Natural Carbon
Synthetic carbon is produced through industrial processes such as chemical vapor deposition or pyrolysis, allowing precise control over purity, structure, and properties for applications in electronics and advanced materials. Natural carbon forms organically over millions of years in geological environments, commonly found as graphite, coal, and diamond, offering unique crystalline structures and varying degrees of purity influenced by natural conditions. Both types play critical roles in technology and industry, with synthetic carbon enabling tailored performance and natural carbon providing abundant, naturally occurring material sources.
Defining Synthetic Carbon
Synthetic carbon refers to carbon materials engineered through controlled chemical and physical processes, resulting in tailored properties for specific industrial applications. Unlike natural carbon, derived from organic matter undergoing geological transformations, synthetic carbon can exhibit enhanced purity, uniformity, and structural configurations such as carbon nanotubes, graphene, and activated carbon. These engineered forms enable advancements in electronics, energy storage, and environmental technologies by offering superior conductivity, strength, and surface area compared to their natural counterparts.
What is Natural Carbon?
Natural carbon is a form of carbon that occurs organically in the environment, primarily derived from biological sources such as plants, animals, and fossilized remains. It exists in various allotropes including graphite, diamond, and amorphous carbon found in soil and sediments. This organic carbon plays a crucial role in the global carbon cycle, influencing ecosystems, climate regulation, and carbon sequestration processes.
Sources of Synthetic Carbon
Synthetic carbon is primarily produced through industrial processes such as chemical vapor deposition and thermal decomposition of hydrocarbons, leading to materials like synthetic graphite and carbon black. These sources enable precise control over purity and structure, essential for applications in electronics, batteries, and composites. Unlike natural carbon, synthetic carbon often originates from fossil fuels or biomass-derived precursors, highlighting its dependence on controlled manufacturing environments.
Origins of Natural Carbon
Natural carbon originates from ancient biological processes where organic matter, such as plants and marine organisms, decomposed over millions of years under high pressure and temperature, forming fossil fuels like coal, oil, and natural gas. This carbon is embedded in geological formations and plays a critical role in the Earth's carbon cycle, influencing climate regulation and ecosystem dynamics. Unlike synthetic carbon, which is human-made through chemical processes, natural carbon reservoirs provide a long-term carbon storage solution essential for maintaining atmospheric CO2 balance.
Key Differences Between Synthetic and Natural Carbon
Synthetic carbon is engineered through industrial processes such as chemical vapor deposition or graphitization, resulting in highly controlled purity, structure, and properties tailored for specific applications. Natural carbon originates from geological formations like coal, graphite, or diamonds, with variable composition and impurities influenced by environmental factors over millions of years. Key differences include synthetic carbon's consistent quality and customizable characteristics versus natural carbon's variability and naturally occurring isotopic profiles.
Physical and Chemical Properties Comparison
Synthetic carbon typically exhibits a more uniform structure with higher purity and fewer impurities compared to natural carbon, resulting in enhanced mechanical strength and electrical conductivity. Natural carbon, such as graphite or coal, contains varying levels of impurities and heterogeneous microstructures, influencing its thermal stability and chemical reactivity. Chemically, synthetic carbon can be engineered to possess specific surface functionalities and pore sizes, whereas natural carbon's properties are largely dictated by its geological formation and inherent mineral content.
Industrial Applications: Synthetic vs Natural Carbon
Synthetic carbon, produced through controlled processes like chemical vapor deposition, offers precise structural properties ideal for high-performance industrial applications such as aerospace components and electronics. Natural carbon, including graphite and coal-derived forms, remains essential in industries requiring bulk material, such as steel manufacturing and energy production, due to its availability and cost-effectiveness. Industrial applications leverage synthetic carbon for superior purity and tailored characteristics, while natural carbon dominates in large-scale, cost-sensitive operations.
Environmental Impact Analysis
Synthetic carbon production typically relies on fossil fuel-based feedstocks and energy-intensive processes, generating higher greenhouse gas emissions compared to natural carbon sources like biomass. Natural carbon, derived from renewable organic matter, offers a lower carbon footprint by promoting carbon sequestration and reducing reliance on non-renewable resources. Lifecycle assessments reveal that synthetic carbon materials often contribute to greater environmental degradation, whereas natural carbon supports sustainability through soil enrichment and biodiversity preservation.
Future Trends in Carbon Material Innovation
Synthetic carbon materials, such as graphene and carbon nanotubes, are driving future trends in carbon innovation due to their exceptional strength, conductivity, and versatility in applications like electronics and energy storage. Natural carbon forms like graphite and charcoal remain essential for traditional uses but are increasingly supplemented by engineered carbon designed for specific performance enhancements. Emerging research focuses on hybrid carbon materials that combine synthetic precision with natural carbon's sustainability, aiming to revolutionize industries from automotive to aerospace.
Synthetic Carbon vs Natural Carbon Infographic
 materialdif.com
materialdif.com