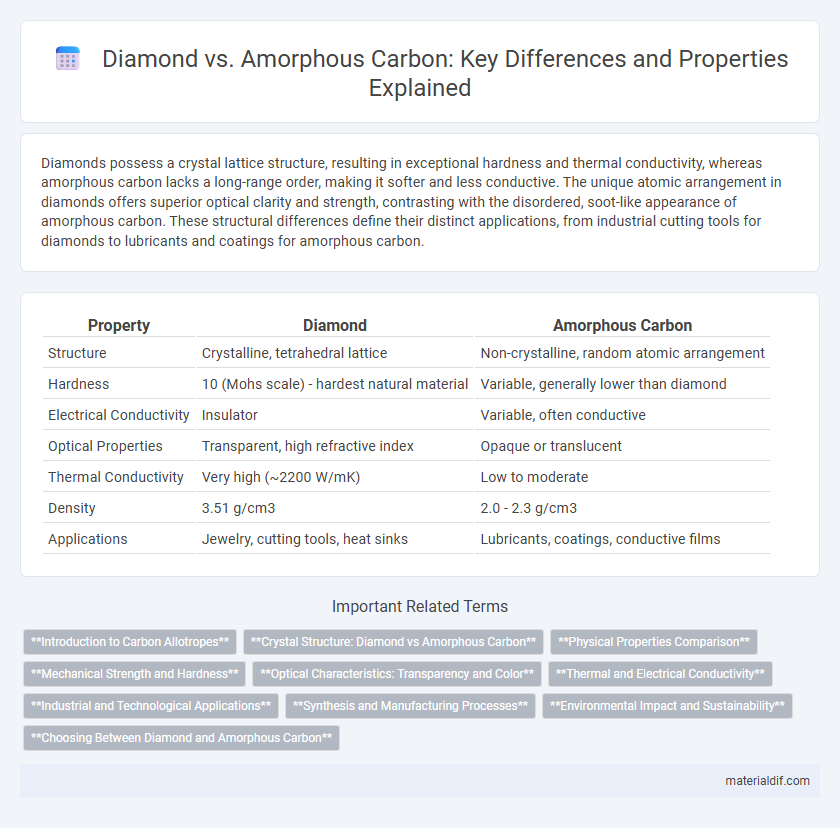
Diamonds possess a crystal lattice structure, resulting in exceptional hardness and thermal conductivity, whereas amorphous carbon lacks a long-range order, making it softer and less conductive. The unique atomic arrangement in diamonds offers superior optical clarity and strength, contrasting with the disordered, soot-like appearance of amorphous carbon. These structural differences define their distinct applications, from industrial cutting tools for diamonds to lubricants and coatings for amorphous carbon.
Table of Comparison
| Property | Diamond | Amorphous Carbon |
|---|---|---|
| Structure | Crystalline, tetrahedral lattice | Non-crystalline, random atomic arrangement |
| Hardness | 10 (Mohs scale) - hardest natural material | Variable, generally lower than diamond |
| Electrical Conductivity | Insulator | Variable, often conductive |
| Optical Properties | Transparent, high refractive index | Opaque or translucent |
| Thermal Conductivity | Very high (~2200 W/mK) | Low to moderate |
| Density | 3.51 g/cm3 | 2.0 - 2.3 g/cm3 |
| Applications | Jewelry, cutting tools, heat sinks | Lubricants, coatings, conductive films |
Introduction to Carbon Allotropes
Carbon allotropes exhibit varied atomic structures shaping their distinct physical properties, with diamond and amorphous carbon serving as prime examples. Diamond features a crystalline lattice of sp3-hybridized carbon atoms, yielding exceptional hardness and high thermal conductivity. In contrast, amorphous carbon lacks a long-range order, consisting of a random mixture of sp2 and sp3 bonds that result in variable properties including electrical conductivity and mechanical flexibility.
Crystal Structure: Diamond vs Amorphous Carbon
Diamond exhibits a highly ordered crystal structure characterized by a cubic lattice where each carbon atom is tetrahedrally bonded to four other atoms, resulting in exceptional hardness and thermal conductivity. Amorphous carbon lacks this long-range order, consisting of a random network of carbon atoms with varying hybridizations, which gives it contrasting electrical and mechanical properties compared to diamond. The differences in crystal structure directly influence their optical transparency, with diamond being transparent and amorphous carbon typically opaque.
Physical Properties Comparison
Diamonds possess a crystalline lattice structure resulting in exceptional hardness, high thermal conductivity, and optical transparency, whereas amorphous carbon lacks a defined crystal structure, leading to variable hardness, lower thermal conductivity, and opaque appearance. The physical properties of diamonds, such as a Mohs hardness of 10 and a density around 3.5 g/cm3, contrast sharply with amorphous carbon's irregular bonding and densities ranging from 1.8 to 2.1 g/cm3. These differences in atomic arrangement account for the superior mechanical strength and optical clarity of diamonds compared to the more versatile but less structurally rigid amorphous carbon materials.
Mechanical Strength and Hardness
Diamonds exhibit exceptional mechanical strength and hardness due to their crystal lattice structure, ranking 10 on the Mohs scale, making them the hardest natural material known. Amorphous carbon lacks a long-range ordered structure, resulting in lower hardness and mechanical stability compared to diamond, with properties varying widely depending on density and bonding configurations. The sp3 hybridization in diamond contributes to its rigidity and resistance to deformation, whereas amorphous carbon contains a mixture of sp2 and sp3 bonds, reducing its overall hardness and mechanical resilience.
Optical Characteristics: Transparency and Color
Diamonds exhibit exceptional transparency and brilliance, characterized by their high refractive index (approximately 2.42) and strong dispersion, which create their signature sparkle and partial colorlessness. In contrast, amorphous carbon lacks a crystalline structure, resulting in opaque or translucent appearances with dark, often black or brown hues due to the random arrangement of carbon atoms absorbing a broad spectrum of light. The distinct optical properties between diamond and amorphous carbon stem from their atomic organization, critically influencing their applications in gemology and optical materials.
Thermal and Electrical Conductivity
Diamond exhibits exceptional thermal conductivity, reaching up to 2000 W/m*K, due to its highly ordered crystal lattice that facilitates efficient phonon transport. In contrast, amorphous carbon lacks this organized structure, resulting in significantly lower thermal conductivity often below 2 W/m*K. Electrically, diamond is an excellent insulator with a wide bandgap of about 5.5 eV, while amorphous carbon can demonstrate variable electrical conductivity depending on its sp2/sp3 bonding ratios, often behaving as a semiconductor or even a conductor.
Industrial and Technological Applications
Diamond's exceptional hardness, thermal conductivity, and optical properties make it indispensable in cutting tools, abrasives, and advanced optics within industrial applications. Amorphous carbon, including forms like activated carbon and carbon black, is widely used for filtration, energy storage in batteries, and as a reinforcing material in composites due to its high surface area and electrical conductivity. Technological advancements leverage diamond coatings for wear resistance and heat dissipation in electronics, while amorphous carbon materials enhance electrodes, catalysts, and supercapacitors in energy and environmental technologies.
Synthesis and Manufacturing Processes
Diamond synthesis typically involves high-pressure high-temperature (HPHT) methods or chemical vapor deposition (CVD) techniques, which create a crystalline lattice structure with exceptional hardness and thermal conductivity. Amorphous carbon, produced by processes such as chemical vapor deposition or pyrolysis of hydrocarbons, lacks long-range order and is characterized by its disordered, non-crystalline structure. The manufacturing of diamond requires precise control of temperature, pressure, and gas composition to ensure crystal growth, while amorphous carbon synthesis focuses on controlling deposition parameters to achieve desired electrical and mechanical properties.
Environmental Impact and Sustainability
Diamonds, especially those mined traditionally, often involve significant environmental degradation, including habitat destruction and high carbon emissions, whereas amorphous carbon materials are typically synthesized with lower ecological footprints. Laboratory-produced amorphous carbon can be engineered from waste products or renewable sources, promoting sustainability through recycling and reduced mining pressures. The push for synthetic diamond alternatives highlights efforts to minimize water usage, energy consumption, and carbon dioxide emissions throughout production cycles.
Choosing Between Diamond and Amorphous Carbon
Diamond exhibits a crystalline structure with exceptional hardness, thermal conductivity, and optical properties, making it ideal for industrial cutting tools and high-performance electronics. Amorphous carbon, lacking a defined crystal structure, offers versatility with its lower density, electrical conductivity, and chemical stability, suited for coatings, batteries, and lubricants. Choosing between diamond and amorphous carbon depends on application-specific requirements such as hardness, conductivity, and cost-efficiency.
Diamond vs Amorphous Carbon Infographic
 materialdif.com
materialdif.com