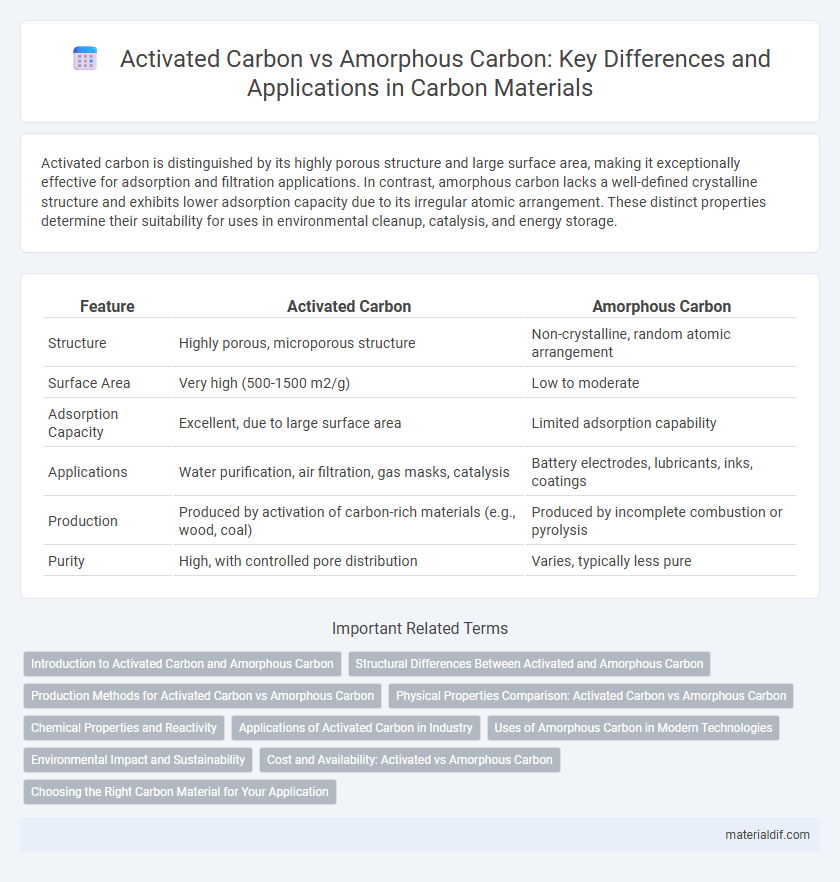
Activated carbon is distinguished by its highly porous structure and large surface area, making it exceptionally effective for adsorption and filtration applications. In contrast, amorphous carbon lacks a well-defined crystalline structure and exhibits lower adsorption capacity due to its irregular atomic arrangement. These distinct properties determine their suitability for uses in environmental cleanup, catalysis, and energy storage.
Table of Comparison
| Feature | Activated Carbon | Amorphous Carbon |
|---|---|---|
| Structure | Highly porous, microporous structure | Non-crystalline, random atomic arrangement |
| Surface Area | Very high (500-1500 m2/g) | Low to moderate |
| Adsorption Capacity | Excellent, due to large surface area | Limited adsorption capability |
| Applications | Water purification, air filtration, gas masks, catalysis | Battery electrodes, lubricants, inks, coatings |
| Production | Produced by activation of carbon-rich materials (e.g., wood, coal) | Produced by incomplete combustion or pyrolysis |
| Purity | High, with controlled pore distribution | Varies, typically less pure |
Introduction to Activated Carbon and Amorphous Carbon
Activated carbon is a highly porous material derived from carbonaceous sources, known for its extensive surface area and adsorption capabilities, widely used in water purification and air filtration. Amorphous carbon lacks a crystalline structure and includes materials like carbon black and charcoal, characterized by disordered atomic arrangements and varied surface chemistry. The distinctive porosity and surface properties of activated carbon contrast with the less ordered structure and lower adsorption efficiency of amorphous carbon, influencing their industrial applications.
Structural Differences Between Activated and Amorphous Carbon
Activated carbon features a highly porous structure with a large surface area due to its extensive network of microscopic pores, enhancing adsorption capabilities. In contrast, amorphous carbon lacks a well-defined lattice or crystallinity, characterized by a random arrangement of carbon atoms and minimal pore development. These structural differences result in activated carbon's superior effectiveness in filtration and adsorption compared to the less porous, disordered morphology of amorphous carbon.
Production Methods for Activated Carbon vs Amorphous Carbon
Activated carbon is produced primarily through physical activation involving carbonization of precursor materials like coconut shells or coal followed by oxidation at high temperatures, or chemical activation using agents such as phosphoric acid or potassium hydroxide to create a porous structure. Amorphous carbon is typically synthesized via pyrolysis of organic compounds or chemical vapor deposition, resulting in a non-crystalline structure without the extensive porosity characteristic of activated carbon. The distinctive production methods influence the surface area, porosity, and adsorption capabilities, with activated carbon optimized for filtration and purification applications while amorphous carbon finds use in coatings and electronic materials.
Physical Properties Comparison: Activated Carbon vs Amorphous Carbon
Activated carbon exhibits a highly porous structure with a large surface area ranging from 500 to 1500 m2/g, making it ideal for adsorption applications, whereas amorphous carbon has a non-crystalline, more compact form with significantly lower surface area, typically below 100 m2/g. The density of activated carbon is generally lower (0.4-0.6 g/cm3) due to its porous nature, in contrast to amorphous carbon which has a higher density around 1.8-2.1 g/cm3. Porosity and surface functional groups in activated carbon contribute to its significant adsorption capacity and catalytic activity, features that are minimal or absent in amorphous carbon.
Chemical Properties and Reactivity
Activated carbon exhibits a highly porous structure with extensive surface area, enhancing its chemical reactivity and adsorption capacity due to abundant functional groups like hydroxyls, carboxyls, and carbonyls. Amorphous carbon lacks the ordered graphite lattice, resulting in lower surface area and fewer reactive sites, which limits its chemical interactions and adsorption efficiency. The diverse surface chemistry of activated carbon facilitates catalytic reactions and pollutant trapping, making it chemically more versatile than amorphous carbon.
Applications of Activated Carbon in Industry
Activated carbon's porous structure enables superior adsorption capabilities, making it essential in water purification, air filtration, and chemical processing industries. Its high surface area allows efficient removal of contaminants, toxins, and organic compounds from liquids and gases. Industries such as wastewater treatment, food and beverage production, and pharmaceutical manufacturing rely heavily on activated carbon for purification and deodorization processes.
Uses of Amorphous Carbon in Modern Technologies
Amorphous carbon's unique disordered atomic structure makes it invaluable in modern technologies such as thin-film coatings for electronics, battery electrodes, and supercapacitors due to its excellent electrical conductivity and chemical stability. Unlike activated carbon, which is primarily used for filtration and adsorption, amorphous carbon plays a critical role in advanced energy storage devices and protective layers in microelectronics. Its versatility enhances performance in semiconductor devices, lithium-ion batteries, and fuel cells, driving innovation in sustainable energy solutions.
Environmental Impact and Sustainability
Activated carbon's porous structure enables high adsorption capacities, making it effective for water and air purification, thus significantly reducing environmental pollutants. In contrast, amorphous carbon, lacking a defined structure, offers limited adsorption and lower efficiency in pollution control applications. Sustainable production of activated carbon often utilizes renewable biomass, enhancing its environmental benefits compared to conventional fossil-fuel-derived amorphous carbon.
Cost and Availability: Activated vs Amorphous Carbon
Activated carbon typically incurs higher costs due to its complex manufacturing process involving raw material activation and high-temperature treatment, whereas amorphous carbon is more cost-effective given its simpler production from less refined sources. Availability of activated carbon is more limited and dependent on industrial supply chains, while amorphous carbon is widely accessible as a byproduct from various combustion and decomposition processes. Market demand for activated carbon in purification and filtration applications often drives price premiums compared to the abundant and readily available amorphous carbon used in general carbon-based materials.
Choosing the Right Carbon Material for Your Application
Activated carbon offers a highly porous structure with a large surface area, making it ideal for applications requiring adsorption, such as water purification and air filtration. In contrast, amorphous carbon exhibits a disordered structure with lower porosity, suited for uses in electrodes and lubricants where conductivity and chemical stability are important. Selecting the right carbon material depends on the specific performance needs, with activated carbon favored for maximum adsorption capacity and amorphous carbon preferred for mechanical strength and electrical applications.
Activated carbon vs Amorphous carbon Infographic
 materialdif.com
materialdif.com