Pyrolytic carbon features a layered, graphitic structure with high mechanical strength and excellent biocompatibility, making it suitable for medical implants and high-temperature applications. Glassy carbon, on the other hand, possesses a non-graphitizing, isotropic structure, offering superior chemical resistance, low density, and high thermal stability for use in electrochemical electrodes and harsh chemical environments. Both materials excel in durability, but their distinct microstructures determine their specialized industrial and biomedical roles.
Table of Comparison
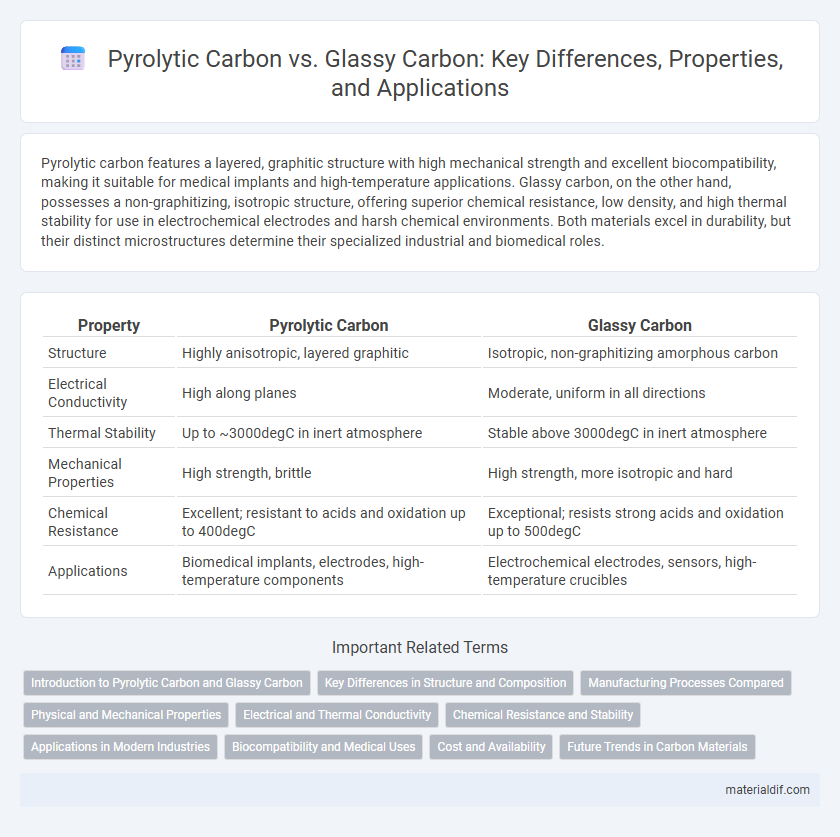
| Property | Pyrolytic Carbon | Glassy Carbon |
|---|---|---|
| Structure | Highly anisotropic, layered graphitic | Isotropic, non-graphitizing amorphous carbon |
| Electrical Conductivity | High along planes | Moderate, uniform in all directions |
| Thermal Stability | Up to ~3000degC in inert atmosphere | Stable above 3000degC in inert atmosphere |
| Mechanical Properties | High strength, brittle | High strength, more isotropic and hard |
| Chemical Resistance | Excellent; resistant to acids and oxidation up to 400degC | Exceptional; resists strong acids and oxidation up to 500degC |
| Applications | Biomedical implants, electrodes, high-temperature components | Electrochemical electrodes, sensors, high-temperature crucibles |
Introduction to Pyrolytic Carbon and Glassy Carbon
Pyrolytic carbon is a high-purity, biocompatible material produced through chemical vapor deposition, exhibiting excellent mechanical strength and thermal stability. Glassy carbon is an amorphous carbon form characterized by its impermeability, chemical resistance, and high thermal conductivity, often used in electrochemical applications. Both materials are distinguished by their microstructure and fabrication processes, influencing their performance in medical, chemical, and electronic industries.
Key Differences in Structure and Composition
Pyrolytic carbon features a layered, graphitic structure with high anisotropy, formed through chemical vapor deposition, resulting in a semi-crystalline composition. Glassy carbon exhibits an isotropic, amorphous structure composed of interconnected graphene-like sheets, producing a non-graphitizing carbon with high chemical resistance. The primary difference lies in pyrolytic carbon's ordered, directional layers versus the random, cross-linked network typical of glassy carbon.
Manufacturing Processes Compared
Pyrolytic carbon is produced through chemical vapor deposition (CVD) where hydrocarbon gases decompose at high temperatures onto substrates, resulting in highly oriented layers with anisotropic properties. In contrast, glassy carbon manufacturing involves the carbonization of polymer precursors, such as phenolic resins, at elevated temperatures in an inert atmosphere, yielding an isotropic, glass-like structure with low porosity. The distinct fabrication methods cause pyrolytic carbon to exhibit superior mechanical strength and tailored electrical conductivity, while glassy carbon is favored for its chemical inertness and thermal stability.
Physical and Mechanical Properties
Pyrolytic carbon exhibits high strength and excellent wear resistance due to its layered graphite-like structure, making it highly anisotropic in mechanical properties. Glassy carbon, characterized by its isotropic and amorphous nature, combines high hardness with low density and outstanding chemical inertness, providing superior thermal stability. The distinct microstructures result in pyrolytic carbon having greater tensile strength along basal planes, while glassy carbon offers uniform compressive strength and enhanced fracture toughness.
Electrical and Thermal Conductivity
Pyrolytic carbon exhibits high anisotropic electrical and thermal conductivity due to its layered graphitic structure, making it highly efficient along the basal planes but less conductive perpendicularly. Glassy carbon, by contrast, has isotropic and comparatively lower electrical and thermal conductivity, attributed to its amorphous, non-graphitic microstructure. The enhanced conductivity of pyrolytic carbon is advantageous in applications requiring directional heat and electron transfer, whereas glassy carbon is preferred for chemical stability and uniform performance.
Chemical Resistance and Stability
Pyrolytic carbon exhibits exceptional chemical resistance and thermal stability due to its layered graphite-like structure, making it highly resistant to corrosion in harsh chemical environments. Glassy carbon offers superior chemical inertness and excellent resistance to oxidation up to high temperatures, attributed to its amorphous, non-porous carbon matrix. Both materials maintain stability under extreme conditions, but glassy carbon generally provides enhanced resistance against strong acids and oxidizing agents compared to pyrolytic carbon.
Applications in Modern Industries
Pyrolytic carbon exhibits exceptional wear resistance and biocompatibility, making it ideal for medical implants and aerospace components where durability under extreme conditions is critical. Glassy carbon's high chemical inertness and electrical conductivity drive its use in electrochemical electrodes, fuel cells, and semiconductor technology. Both materials optimize performance by combining unique structural properties with industrial demands for reliability and efficiency in cutting-edge applications.
Biocompatibility and Medical Uses
Pyrolytic carbon exhibits excellent biocompatibility due to its high wear resistance and chemical stability, making it ideal for heart valves and orthopedic implants. Glassy carbon, with its unique inertness and electrical conductivity, is favored for neural interfaces and biosensors in medical devices. Both materials are crucial in advanced biomedical applications, offering durability and minimal tissue reaction.
Cost and Availability
Pyrolytic carbon is generally more expensive than glassy carbon due to its complex manufacturing process involving chemical vapor deposition, which also limits its availability to specialized suppliers. Glassy carbon, produced through the controlled pyrolysis of polymers, is more widely available and cost-effective for bulk applications. The balance of cost versus performance often guides selection, with pyrolytic carbon favored in high-precision technologies and glassy carbon preferred for general industrial use.
Future Trends in Carbon Materials
Pyrolytic carbon offers high thermal stability and anisotropic properties ideal for advanced engineering applications, while glassy carbon exhibits exceptional chemical resistance and electrical conductivity suited to electrochemical devices. Emerging trends emphasize hybrid carbon materials combining the structural benefits of pyrolytic carbon with the surface chemistry advantages of glassy carbon for energy storage and sensor technologies. Advances in nanostructuring and scalable synthesis are expected to drive innovation in carbon-based composites, targeting improved durability, conductivity, and multifunctionality in future material solutions.
Pyrolytic Carbon vs Glassy Carbon Infographic
 materialdif.com
materialdif.com