Tin corrosion occurs when tin reacts with environmental elements like moisture and oxygen, leading to the gradual deterioration of the tin surface. Galvanic corrosion arises from electrochemical reactions between tin and a different metal in electrical contact, accelerating corrosion due to the potential difference. Understanding the distinction between tin corrosion and galvanic corrosion is crucial for selecting appropriate protective measures in metal applications.
Table of Comparison
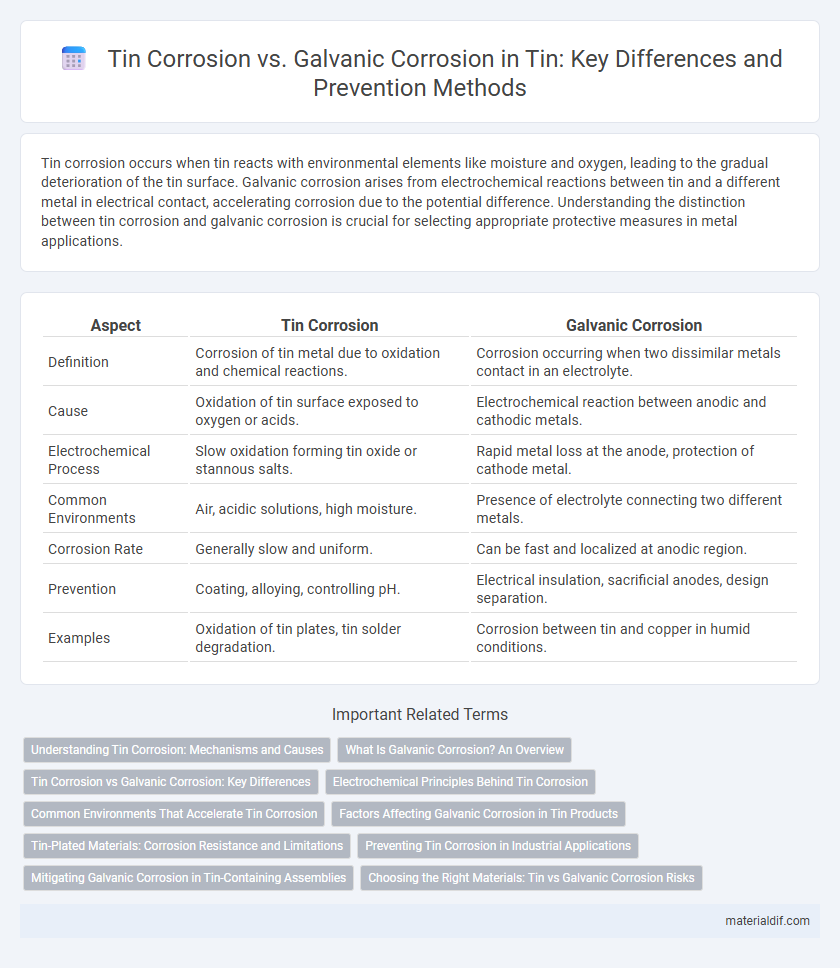
| Aspect | Tin Corrosion | Galvanic Corrosion |
|---|---|---|
| Definition | Corrosion of tin metal due to oxidation and chemical reactions. | Corrosion occurring when two dissimilar metals contact in an electrolyte. |
| Cause | Oxidation of tin surface exposed to oxygen or acids. | Electrochemical reaction between anodic and cathodic metals. |
| Electrochemical Process | Slow oxidation forming tin oxide or stannous salts. | Rapid metal loss at the anode, protection of cathode metal. |
| Common Environments | Air, acidic solutions, high moisture. | Presence of electrolyte connecting two different metals. |
| Corrosion Rate | Generally slow and uniform. | Can be fast and localized at anodic region. |
| Prevention | Coating, alloying, controlling pH. | Electrical insulation, sacrificial anodes, design separation. |
| Examples | Oxidation of tin plates, tin solder degradation. | Corrosion between tin and copper in humid conditions. |
Understanding Tin Corrosion: Mechanisms and Causes
Tin corrosion primarily occurs through oxidation when exposed to moisture and air, leading to the formation of a dull, white oxide layer known as tin oxide. Unlike galvanic corrosion, which results from the electrochemical reaction between dissimilar metals in contact with an electrolyte, tin corrosion is a uniform chemical degradation process. Understanding the specific environmental factors, such as humidity, temperature, and presence of acidic or alkaline agents, is crucial to mitigating tin corrosion and ensuring the longevity of tin-coated surfaces.
What Is Galvanic Corrosion? An Overview
Galvanic corrosion occurs when two dissimilar metals, such as tin and copper, are electrically connected in the presence of an electrolyte, causing accelerated corrosion of the more anodic metal, typically tin. This electrochemical process results from differences in electrode potentials, leading to tin acting as the anode and corroding faster than it would through uniform corrosion alone. Understanding the galvanic series and the electrolyte environment is critical in controlling galvanic corrosion and enhancing the longevity of tin-coated components.
Tin Corrosion vs Galvanic Corrosion: Key Differences
Tin corrosion primarily results from exposure to acids, alkalis, and moisture, leading to the gradual degradation of the tin coating. Galvanic corrosion occurs when tin is in electrical contact with a more noble metal in an electrolyte, causing accelerated corrosion of the less noble metal, typically the tin. The key difference lies in the mechanism: tin corrosion is a chemical process affecting the tin layer itself, while galvanic corrosion involves electrochemical interaction between dissimilar metals.
Electrochemical Principles Behind Tin Corrosion
Tin corrosion primarily occurs through electrochemical reactions where tin oxidizes in the presence of moisture and oxygen, forming tin oxides and hydroxides that degrade the metal surface. In contrast, galvanic corrosion involving tin arises when tin is coupled with a more noble metal, creating a galvanic cell where tin acts as the anode and corrodes preferentially due to differences in electrode potentials. The electrochemical principles governing tin corrosion emphasize its anodic dissolution driven by redox reactions, influenced by factors such as electrolyte composition, pH, and electrical conductivity.
Common Environments That Accelerate Tin Corrosion
Tin corrosion is commonly accelerated in acidic or alkaline environments, such as those containing hydrochloric acid or sodium hydroxide, which break down the protective oxide layer on tin surfaces. In contrast, galvanic corrosion occurs when tin is electrically coupled with a more noble metal in the presence of an electrolyte, like seawater or humid air, causing tin to act as the anode and corrode faster. High humidity, chloride ions, and elevated temperatures significantly increase the rate of both tin corrosion and galvanic corrosion, impacting applications in electronics and packaging.
Factors Affecting Galvanic Corrosion in Tin Products
Factors affecting galvanic corrosion in tin products include the electrochemical potential difference between tin and the coupled metal, the conductivity of the electrolyte, and the surface area ratio of the metals involved. High conductivity electrolytes accelerate ion transfer, intensifying corrosion rates, while a larger cathodic area relative to the anodic tin surface increases localized corrosion. Temperature and pH of the environment also critically influence galvanic interactions, often worsening corrosion in acidic or elevated temperature conditions.
Tin-Plated Materials: Corrosion Resistance and Limitations
Tin-plated materials exhibit strong corrosion resistance primarily due to tin's ability to form a protective oxide layer that inhibits base metal degradation. However, when in contact with dissimilar metals, galvanic corrosion can occur, accelerating deterioration at the interface between tin and the less noble metal. Understanding the electrochemical potential differences is crucial for mitigating galvanic corrosion in tin-plated assemblies and ensuring longevity in applications like electronics and food packaging.
Preventing Tin Corrosion in Industrial Applications
Tin corrosion primarily results from tin oxide formation in humid or acidic environments, compromising protective coatings. Galvanic corrosion occurs when tin contacts a more noble metal, accelerating deterioration through electrochemical reactions. Preventing tin corrosion in industrial applications involves using appropriate alloy compositions, applying protective coatings such as nickel or organic inhibitors, and controlling environmental factors like pH and humidity.
Mitigating Galvanic Corrosion in Tin-Containing Assemblies
Mitigating galvanic corrosion in tin-containing assemblies requires controlling the electrochemical potential differences between tin and adjacent metals such as copper or steel. Applying protective coatings, using insulating barriers, and ensuring uniform tin plating thickness minimizes galvanic coupling and reduces localized corrosion rates. Proper design considerations, including avoiding direct contact between dissimilar metals and maintaining low moisture environments, significantly extend the service life of tin-coated components.
Choosing the Right Materials: Tin vs Galvanic Corrosion Risks
Tin offers excellent corrosion resistance in many environments due to its stable oxide layer, making it a preferred choice for protective coatings on steel and copper. When paired with dissimilar metals, galvanic corrosion risks increase, especially if tin is combined with more noble metals like copper or silver, which can accelerate tin's corrosion. Selecting materials with similar electrode potentials minimizes galvanic corrosion, ensuring tin coatings remain effective in safeguarding underlying metals.
Tin corrosion vs Galvanic corrosion Infographic
 materialdif.com
materialdif.com