Polystyrene offers excellent rigidity and insulation properties, making it ideal for disposable cups and food containers, while PET (polyethylene terephthalate) provides superior clarity, strength, and recyclability, often used in beverage bottles and packaging. PET's chemical resistance and lightweight nature contribute to its widespread adoption in sustainable packaging solutions. Choosing between polystyrene and PET depends on the specific application requirements, including environmental impact and product durability.
Table of Comparison
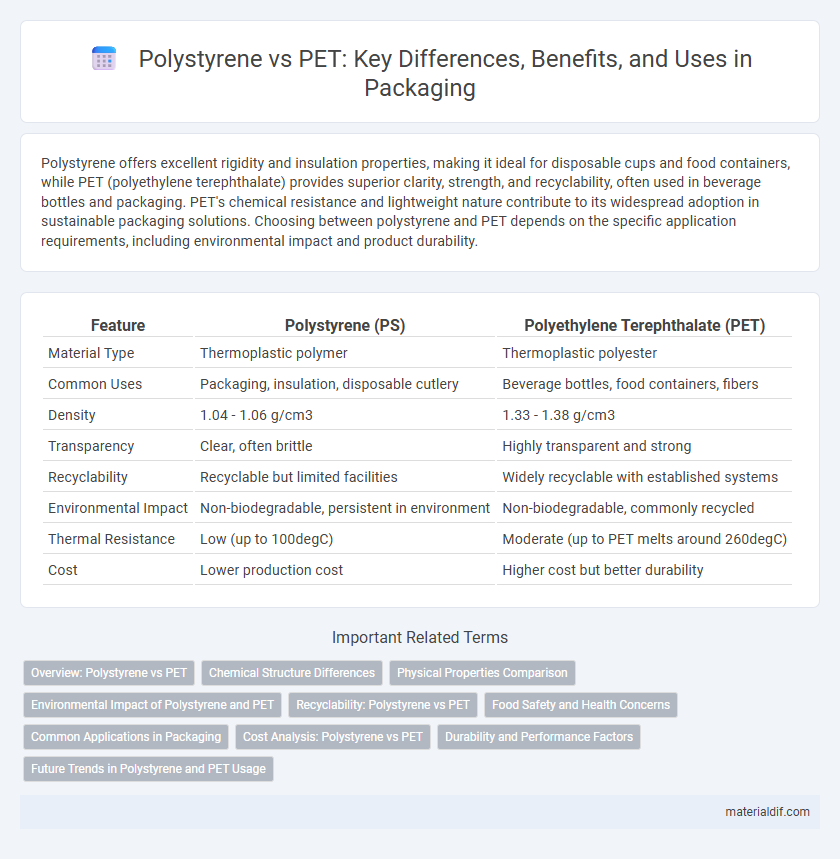
| Feature | Polystyrene (PS) | Polyethylene Terephthalate (PET) |
|---|---|---|
| Material Type | Thermoplastic polymer | Thermoplastic polyester |
| Common Uses | Packaging, insulation, disposable cutlery | Beverage bottles, food containers, fibers |
| Density | 1.04 - 1.06 g/cm3 | 1.33 - 1.38 g/cm3 |
| Transparency | Clear, often brittle | Highly transparent and strong |
| Recyclability | Recyclable but limited facilities | Widely recyclable with established systems |
| Environmental Impact | Non-biodegradable, persistent in environment | Non-biodegradable, commonly recycled |
| Thermal Resistance | Low (up to 100degC) | Moderate (up to PET melts around 260degC) |
| Cost | Lower production cost | Higher cost but better durability |
Overview: Polystyrene vs PET
Polystyrene (PS) is a rigid, lightweight plastic known for its excellent insulation and cushioning properties, commonly used in packaging and disposable items, whereas PET (Polyethylene Terephthalate) is a clear, strong, and recyclable polyester widely utilized in beverage bottles and food containers. PET offers superior barrier properties against moisture and gases, making it ideal for food preservation, while Polystyrene provides better thermal insulation and cost-effectiveness for short-term use. Both materials play distinct roles in packaging industries based on their mechanical properties, recyclability, and environmental impact considerations.
Chemical Structure Differences
Polystyrene (PS) is a polymer consisting of styrene monomers with a phenyl group attached to every other carbon atom in the backbone, imparting rigidity and aromatic characteristics. Polyethylene terephthalate (PET) is a polyester formed by the condensation of ethylene glycol and terephthalic acid, featuring ester functional groups and a repeating aromatic ester linkage. The key chemical structure difference is that PS has a hydrocarbon backbone with pendant phenyl rings, while PET contains ester linkages in its backbone, resulting in distinct physical and chemical properties.
Physical Properties Comparison
Polystyrene exhibits lower density, typically around 1.05 g/cm3, compared to PET's density of approximately 1.38 g/cm3, making polystyrene lighter for packaging applications. Polystyrene has a glass transition temperature near 100degC, which is significantly lower than PET's 70-80degC range, affecting thermal stability and impact resistance. In terms of tensile strength, PET outperforms polystyrene with values around 50-75 MPa, while polystyrene ranges between 30-50 MPa, reflecting PET's superior durability and flexibility.
Environmental Impact of Polystyrene and PET
Polystyrene has a higher environmental impact than PET due to its low recycling rate and non-biodegradable nature, contributing significantly to landfill waste and ocean pollution. PET is more environmentally friendly, with higher recyclability and lower carbon footprint during production and disposal. Both materials pose challenges, but PET's durability and widespread recycling infrastructure make it a preferable option for reducing plastic pollution.
Recyclability: Polystyrene vs PET
Polystyrene, commonly used in packaging and insulation, faces significant challenges in recyclability due to its tendency to break down into small fragments and limited acceptance in curbside recycling programs. PET (Polyethylene Terephthalate) offers superior recyclability with established infrastructure allowing efficient collection, sorting, and processing into new containers and fibers. The high demand for recycled PET in manufacturing contrasts with the lower market value and processing complexity of recycled polystyrene, making PET a more sustainable choice in terms of circular economy practices.
Food Safety and Health Concerns
Polystyrene and PET differ significantly in food safety and health profiles; polystyrene can release styrene monomers, considered potentially carcinogenic, especially when heated or in contact with fatty foods, raising concerns about its use in food containers. PET, or polyethylene terephthalate, is widely regarded as safer for food and beverage packaging due to its inert properties and lower risk of chemical leaching, making it a preferred choice for bottled water and soft drinks. Regulatory agencies like the FDA classify PET as generally recognized as safe (GRAS), while polystyrene is subject to stricter scrutiny due to potential health risks linked to styrene exposure.
Common Applications in Packaging
Polystyrene is widely used for disposable cutlery, food containers, and insulation due to its lightweight and rigid properties, making it ideal for packaging perishable goods. PET (Polyethylene Terephthalate) is preferred for beverage bottles, food jars, and microwaveable trays because of its excellent clarity, strength, and recyclability. Both materials serve critical roles in packaging, with polystyrene excelling in protective cushioning and PET dominating transparent, durable packaging solutions.
Cost Analysis: Polystyrene vs PET
Polystyrene generally offers a lower production cost compared to PET due to its simpler polymerization process and less expensive raw materials, making it a cost-effective option for disposable packaging. PET, however, incurs higher costs driven by its demand for food-grade quality and recycling infrastructure, which can increase overall expenses. When evaluating cost efficiency, Polystyrene is favored for budget-sensitive applications, while PET's durability and recyclability justify its premium pricing in sustainable packaging solutions.
Durability and Performance Factors
Polystyrene exhibits lower impact resistance and flexibility compared to PET, making it less durable under mechanical stress and repeated use. PET offers superior tensile strength, chemical resistance, and thermal stability, which enhances its performance in packaging and industrial applications. The rigidity of polystyrene limits its longevity in high-stress environments, whereas PET maintains integrity over extended periods and varying conditions.
Future Trends in Polystyrene and PET Usage
Future trends in polystyrene and PET usage are driven by increasing demand for sustainable packaging solutions, with PET favored for its superior recyclability and growing adoption in beverage containers. Innovations in bio-based polystyrene and advanced recycling technologies aim to reduce environmental impact, expanding its applications in insulation and disposable goods. Market forecasts project a gradual shift towards PET in food and beverage sectors, while polystyrene retains a niche due to cost-effectiveness and lightweight properties.
Polystyrene vs PET Infographic
 materialdif.com
materialdif.com