Copolymers consist of two or more different monomers chemically bonded, offering a combination of properties that can be tailored for specific applications. Homopolymers are made from a single type of monomer, resulting in uniform structural characteristics and predictable performance. The choice between copolymer and homopolymer depends on the desired balance of mechanical strength, flexibility, and chemical resistance in the final product.
Table of Comparison
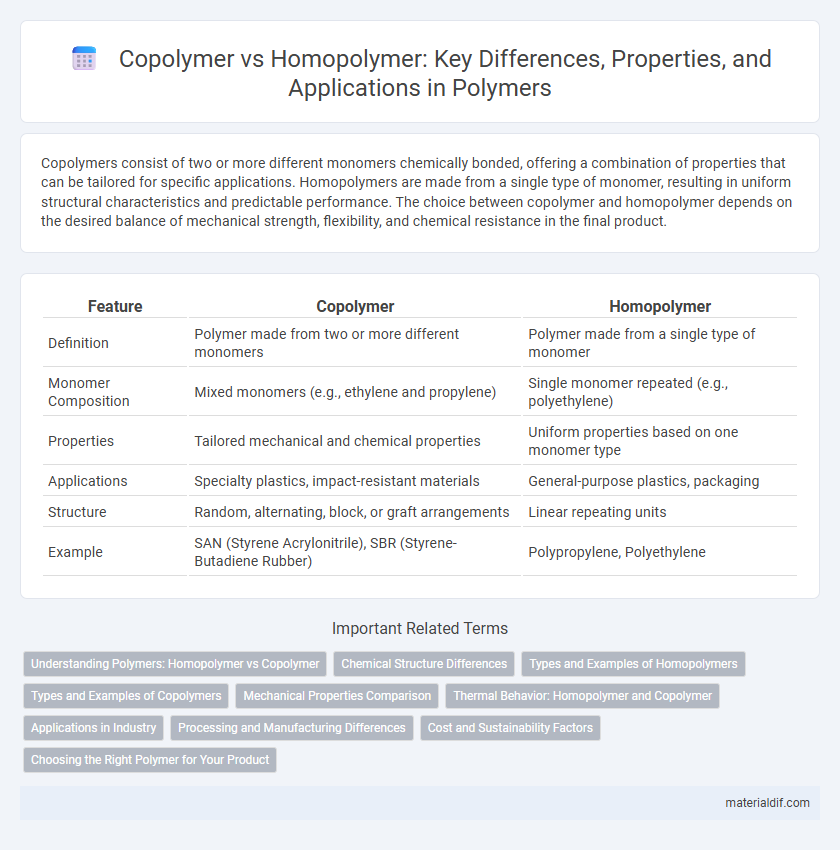
| Feature | Copolymer | Homopolymer |
|---|---|---|
| Definition | Polymer made from two or more different monomers | Polymer made from a single type of monomer |
| Monomer Composition | Mixed monomers (e.g., ethylene and propylene) | Single monomer repeated (e.g., polyethylene) |
| Properties | Tailored mechanical and chemical properties | Uniform properties based on one monomer type |
| Applications | Specialty plastics, impact-resistant materials | General-purpose plastics, packaging |
| Structure | Random, alternating, block, or graft arrangements | Linear repeating units |
| Example | SAN (Styrene Acrylonitrile), SBR (Styrene-Butadiene Rubber) | Polypropylene, Polyethylene |
Understanding Polymers: Homopolymer vs Copolymer
Homopolymers consist of repeating units derived from a single type of monomer, resulting in uniform chemical properties and predictable mechanical behavior. Copolymers are composed of two or more different monomers, which allows for tailored material characteristics such as improved flexibility, impact resistance, or chemical resistance. Understanding the structural differences between homopolymers and copolymers is crucial for selecting the appropriate polymer for applications ranging from packaging to automotive components.
Chemical Structure Differences
Copolymer consists of two or more different monomer units chemically bonded in the polymer chain, creating a heterogeneous molecular structure that alters physical and chemical properties. Homopolymer is formed from a single type of monomer, resulting in a uniform repeating unit along the polymer backbone. These structural differences influence attributes such as flexibility, thermal resistance, and chemical reactivity in various applications.
Types and Examples of Homopolymers
Homopolymers consist of repeating units derived from a single type of monomer, such as polyethylene (PE), polystyrene (PS), and polyvinyl chloride (PVC). Types of homopolymers include linear, branched, and cross-linked polymers, each exhibiting distinct physical properties and applications. Common examples showcase their use in packaging, automotive parts, and construction materials due to uniformity in molecular structure and predictable performance.
Types and Examples of Copolymers
Copolymers consist of two or more different monomers combined within the same polymer chain, classified into types such as random, alternating, block, and graft copolymers. Examples include Styrene-butadiene rubber (SBR), used in tires as a block copolymer, and Acrylonitrile butadiene styrene (ABS), a graft copolymer widely used in automotive parts and consumer goods. These variations in copolymer structure enable tailored mechanical and chemical properties not achievable with homopolymers like polyethylene or polystyrene.
Mechanical Properties Comparison
Copolymers exhibit enhanced mechanical properties compared to homopolymers due to the incorporation of two or more monomer types, which improves tensile strength, flexibility, and impact resistance. Homopolymers typically have uniform mechanical characteristics suited for applications requiring consistent rigidity or elasticity. The tailored molecular structure of copolymers allows for optimized performance in demanding mechanical environments, making them preferable for advanced engineering materials.
Thermal Behavior: Homopolymer and Copolymer
Homopolymers exhibit distinct melting points due to their uniform molecular structure, resulting in consistent thermal behavior and predictable melting temperature ranges. Copolymers, composed of two or more different monomer units, show varied thermal transitions depending on the monomer ratio and arrangement, often leading to broadened or multiple melting and glass transition temperatures. This variation in thermal behavior makes copolymers suitable for applications requiring tailored melting points and thermal stability.
Applications in Industry
Copolymer offers enhanced mechanical properties and chemical resistance, making it ideal for automotive parts, packaging films, and medical devices. Homopolymer, known for its uniform polymer chains, is widely used in applications such as plastic containers, fibers, and molded products where consistent performance is essential. Industrial applications leverage copolymers for flexibility and durability, while homopolymers provide cost-effective solutions with reliable strength.
Processing and Manufacturing Differences
Copolymers exhibit enhanced processing versatility due to their tunable composition, allowing manufacturers to optimize melting points, crystallinity, and mechanical properties for specific applications, unlike homopolymers which have uniform thermal and flow characteristics. The manufacturing complexity of copolymers increases as precise control over monomer ratios and sequence distribution is required, often involving more intricate polymerization techniques such as controlled radical or coordination polymerization. Homopolymers typically enable simpler production processes with consistent performance metrics, but lack the property customization that copolymers provide, impacting their adaptability in advanced manufacturing scenarios.
Cost and Sustainability Factors
Copolymers typically offer enhanced material properties compared to homopolymers, which can influence cost-efficiency in applications requiring specific performance criteria. While homopolymers are generally less expensive due to simpler synthesis, copolymers can provide greater sustainability benefits by enabling the use of renewable monomers and improving recyclability. Evaluating both cost and environmental impact reveals that copolymers hold potential for reducing ecological footprints in polymer manufacturing and end-use.
Choosing the Right Polymer for Your Product
Selecting the appropriate polymer depends on the specific application requirements, where copolymers offer enhanced properties by combining two or more monomers, providing improved flexibility, impact resistance, or chemical stability compared to homopolymers made from a single monomer. Copolymers like styrene-butadiene rubber (SBR) exhibit superior toughness and elasticity, making them ideal for automotive tires, while homopolymers such as polypropylene deliver high crystallinity and stiffness suitable for packaging and household goods. Understanding the molecular structure and end-use conditions ensures optimal performance, durability, and cost-effectiveness in polymer product design.
Copolymer vs Homopolymer Infographic
 materialdif.com
materialdif.com