Polyethylene terephthalate (PET) is synthesized through the polymerization of its monomers, primarily ethylene glycol and terephthalic acid. While monomers are small, simple molecules with reactive functional groups, polymers like PET consist of long chains formed by repeating these monomers, giving the material its durable and flexible properties. This transformation from monomer to polymer results in enhanced chemical resistance and mechanical strength, making PET widely used in packaging and textiles.
Table of Comparison
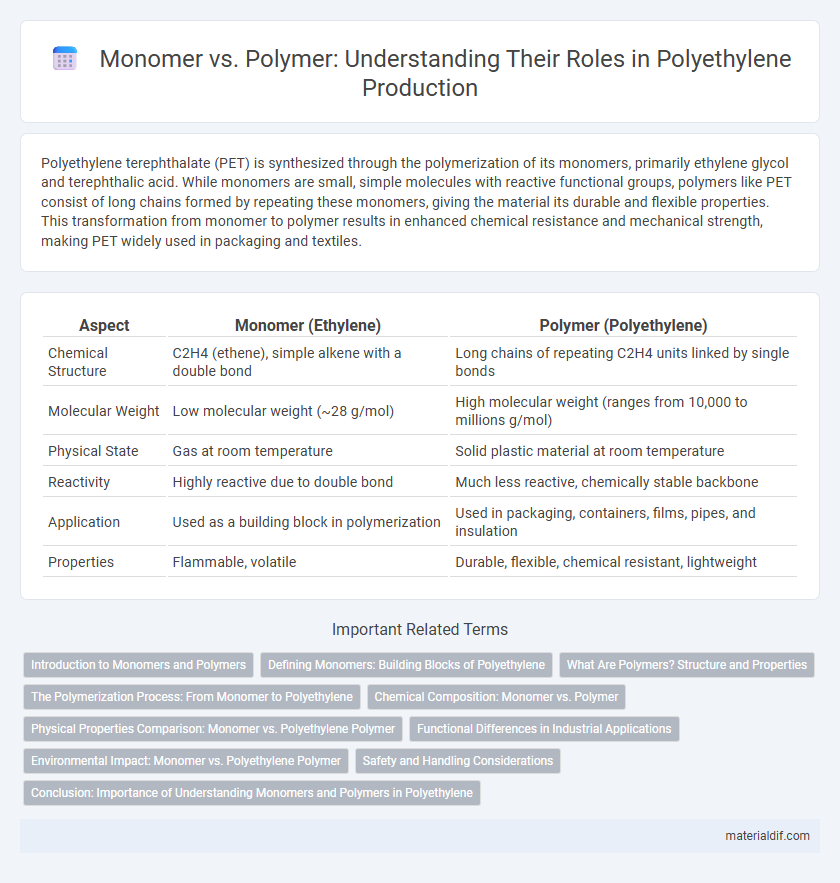
| Aspect | Monomer (Ethylene) | Polymer (Polyethylene) |
|---|---|---|
| Chemical Structure | C2H4 (ethene), simple alkene with a double bond | Long chains of repeating C2H4 units linked by single bonds |
| Molecular Weight | Low molecular weight (~28 g/mol) | High molecular weight (ranges from 10,000 to millions g/mol) |
| Physical State | Gas at room temperature | Solid plastic material at room temperature |
| Reactivity | Highly reactive due to double bond | Much less reactive, chemically stable backbone |
| Application | Used as a building block in polymerization | Used in packaging, containers, films, pipes, and insulation |
| Properties | Flammable, volatile | Durable, flexible, chemical resistant, lightweight |
Introduction to Monomers and Polymers
Monomers are small, repeating molecules that serve as the fundamental building blocks for polymers, including polyethylene. Polyethylene is formed through the polymerization of ethylene monomers, creating long chains that determine its physical properties such as flexibility, strength, and durability. Understanding the relationship between monomers and polymers is essential for manipulating polyethylene characteristics in applications ranging from packaging to industrial materials.
Defining Monomers: Building Blocks of Polyethylene
Monomers are small, reactive molecules that serve as the fundamental building blocks of polyethylene. Ethylene, the primary monomer in polyethylene production, consists of two carbon atoms double-bonded to each other, allowing repeated polymerization. Through a process called addition polymerization, thousands of ethylene monomers chemically bond to form long, stable polyethylene polymer chains, defining its unique properties.
What Are Polymers? Structure and Properties
Polymers are large molecules composed of repeating structural units called monomers, chemically bonded to form long chains or networks. Polyethylene, a common polymer, consists of ethylene monomers linked through addition polymerization, resulting in a structure characterized by high molecular weight and variable branching. This molecular arrangement imparts properties such as durability, flexibility, chemical resistance, and low density, making polyethylene suitable for diverse applications including packaging, containers, and insulation.
The Polymerization Process: From Monomer to Polyethylene
The polymerization process transforms ethylene monomers, small hydrocarbon molecules with the formula C2H4, into long-chain polyethylene polymers through catalytic or thermal methods. This chemical reaction links numerous ethylene units via covalent bonds, creating high-density polyethylene (HDPE) or low-density polyethylene (LDPE) depending on reaction conditions. The resulting polyethylene exhibits diverse molecular weights and branching structures, determining its mechanical properties and applications in packaging, containers, and plastic films.
Chemical Composition: Monomer vs. Polymer
Ethylene, a simple hydrocarbon monomer with the formula C2H4, undergoes polymerization to form polyethylene, a long-chain polymer composed of repeating -CH2- units. The monomer consists of small, reactive ethylene molecules with a double bond that breaks during polymerization, linking the monomers into a stable polymer chain. Polyethylene's chemical composition transforms from discrete C2H4 units into extensive macromolecules with high molecular weight, providing unique physical properties distinct from the monomer.
Physical Properties Comparison: Monomer vs. Polyethylene Polymer
Ethylene monomer is a small, volatile gas with low molecular weight and simple physical properties such as low boiling point and high flammability. In contrast, polyethylene polymer consists of long hydrocarbon chains with high molecular weight, resulting in a solid material characterized by high tensile strength, flexibility, and chemical resistance. The transformation from monomer to polymer significantly enhances physical properties, making polyethylene suitable for diverse applications like packaging, insulation, and molded products.
Functional Differences in Industrial Applications
Ethylene, the monomer of polyethylene, exhibits high reactivity and low molecular weight, allowing precise control in polymerization processes to tailor material properties. Polyethylene, the polymer, offers enhanced mechanical strength, chemical resistance, and flexibility, making it suitable for diverse industrial uses such as packaging films, containers, and pipes. The functional differences between ethylene and polyethylene enable the transformation from a simple gaseous hydrocarbon to versatile solid materials essential in manufacturing and construction.
Environmental Impact: Monomer vs. Polyethylene Polymer
Ethylene monomers are volatile organic compounds that contribute to air pollution and pose toxicity risks during production. Polyethylene polymers, due to their high molecular weight and chemical stability, persist in the environment, leading to long-term plastic pollution and microplastic formation. The polymer's durability complicates degradation, creating significant challenges for waste management and ecosystem health.
Safety and Handling Considerations
Ethylene monomer requires strict safety measures due to its flammability and toxicity, necessitating proper ventilation and explosion-proof equipment during handling. In contrast, polyethylene polymer is stable and non-toxic, posing minimal risk in solid form but requiring caution to avoid dust inhalation during processing or cutting. Both forms demand adherence to safety protocols to prevent accidents and ensure safe industrial operations.
Conclusion: Importance of Understanding Monomers and Polymers in Polyethylene
Understanding the relationship between ethylene monomers and polyethylene polymers is crucial for optimizing manufacturing processes and enhancing material properties like durability and flexibility. Mastery of monomer-polymer interactions enables the development of tailored polyethylene variants for specific applications such as packaging, construction, and medical devices. This knowledge drives innovations in recycling technologies and sustainability efforts by improving polymer breakdown and reuse efficiency.
Monomer vs Polymer Infographic
 materialdif.com
materialdif.com