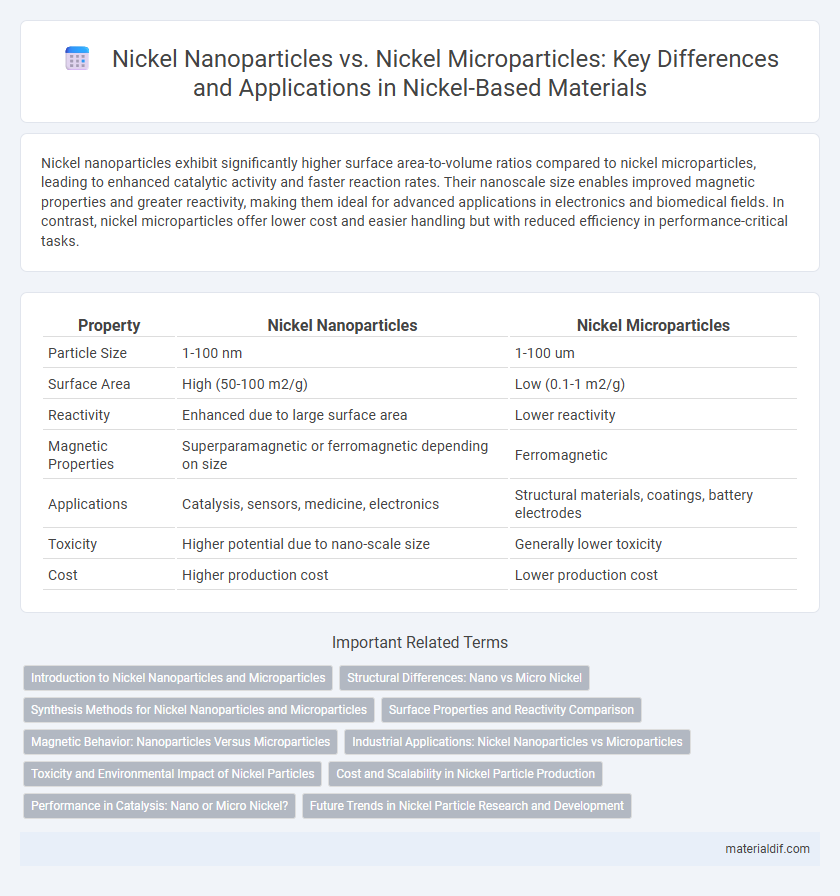
Nickel nanoparticles exhibit significantly higher surface area-to-volume ratios compared to nickel microparticles, leading to enhanced catalytic activity and faster reaction rates. Their nanoscale size enables improved magnetic properties and greater reactivity, making them ideal for advanced applications in electronics and biomedical fields. In contrast, nickel microparticles offer lower cost and easier handling but with reduced efficiency in performance-critical tasks.
Table of Comparison
| Property | Nickel Nanoparticles | Nickel Microparticles |
|---|---|---|
| Particle Size | 1-100 nm | 1-100 um |
| Surface Area | High (50-100 m2/g) | Low (0.1-1 m2/g) |
| Reactivity | Enhanced due to large surface area | Lower reactivity |
| Magnetic Properties | Superparamagnetic or ferromagnetic depending on size | Ferromagnetic |
| Applications | Catalysis, sensors, medicine, electronics | Structural materials, coatings, battery electrodes |
| Toxicity | Higher potential due to nano-scale size | Generally lower toxicity |
| Cost | Higher production cost | Lower production cost |
Introduction to Nickel Nanoparticles and Microparticles
Nickel nanoparticles exhibit significantly higher surface area-to-volume ratios compared to nickel microparticles, enhancing catalytic activity and magnetic properties at the nanoscale. These nanoparticles typically range from 1 to 100 nanometers, while microparticles are sized between 1 to 100 micrometers, influencing their physical and chemical behaviors. Applications in electronics, medicine, and environmental remediation leverage the unique physicochemical features of nickel nanoparticles distinct from conventional microparticles.
Structural Differences: Nano vs Micro Nickel
Nickel nanoparticles exhibit a significantly higher surface area to volume ratio compared to nickel microparticles, resulting in enhanced catalytic activity and chemical reactivity. The crystalline structure of nickel nanoparticles often displays altered lattice parameters due to quantum size effects, whereas microparticles maintain bulk-like crystalline properties with more stable grain boundaries. These structural differences influence physical characteristics such as magnetic behavior, with nanoparticles showing superparamagnetism and microparticles exhibiting ferromagnetism.
Synthesis Methods for Nickel Nanoparticles and Microparticles
Nickel nanoparticles are commonly synthesized using chemical reduction methods, such as hydrazine or sodium borohydride reduction, enabling precise control over particle size and morphology. In contrast, nickel microparticles are typically produced via physical methods like mechanical milling or spray drying, which result in larger, less uniform particles. Advanced techniques like electrodeposition and thermal decomposition are also employed for nickel nanoparticles to achieve high purity and specific surface properties.
Surface Properties and Reactivity Comparison
Nickel nanoparticles exhibit significantly higher surface area-to-volume ratios compared to nickel microparticles, resulting in increased surface energy and enhanced catalytic reactivity. The large surface-to-volume ratio of nickel nanoparticles facilitates more active sites for chemical reactions, improving their performance in applications such as hydrogenation and electrocatalysis. In contrast, nickel microparticles have reduced surface energy and fewer active sites, leading to lower overall reactivity and slower reaction rates.
Magnetic Behavior: Nanoparticles Versus Microparticles
Nickel nanoparticles exhibit superparamagnetic behavior due to their particle size being at the nanoscale, which leads to single-domain magnetic structures and negligible coercivity. In contrast, nickel microparticles, larger in size, display ferromagnetic properties with multi-domain structures, resulting in higher coercivity and remanence. This size-dependent magnetic behavior is critical for applications such as magnetic storage, catalysis, and biomedical imaging.
Industrial Applications: Nickel Nanoparticles vs Microparticles
Nickel nanoparticles exhibit superior catalytic activity and enhanced surface area compared to nickel microparticles, making them ideal for use in hydrogenation reactions and fuel cells in industrial applications. Microparticles are preferred for applications requiring greater mechanical strength, such as electrode manufacturing and corrosion-resistant coatings. The distinct physicochemical properties of nickel nanoparticles and microparticles determine their suitability for specific industrial roles, optimizing performance and cost-efficiency.
Toxicity and Environmental Impact of Nickel Particles
Nickel nanoparticles exhibit significantly higher toxicity compared to nickel microparticles due to their increased surface area and reactivity, which can lead to enhanced cellular uptake and oxidative stress in biological systems. Environmental impact studies indicate that nickel nanoparticles pose greater ecological risks by penetrating soil and water ecosystems more readily, potentially disrupting microbial communities and accumulating in aquatic organisms. In contrast, nickel microparticles demonstrate lower bioavailability and slower environmental mobility, resulting in reduced toxicological effects and environmental persistence.
Cost and Scalability in Nickel Particle Production
Nickel nanoparticles typically incur higher production costs compared to nickel microparticles due to the advanced synthesis methods and strict quality control required. Scalability of nickel microparticle production benefits from established industrial processes, enabling large-volume manufacturing at lower expenses. However, nickel nanoparticles face challenges in scaling up economically, often limiting their widespread commercial application despite superior surface area and reactivity.
Performance in Catalysis: Nano or Micro Nickel?
Nickel nanoparticles exhibit significantly higher catalytic activity compared to nickel microparticles due to their increased surface area-to-volume ratio, offering more active sites for chemical reactions. The enhanced electron transfer and adsorption capabilities of nano-sized nickel particles lead to improved efficiency in processes such as hydrogenation and carbon-carbon coupling reactions. However, nickel microparticles may provide greater stability and ease of recovery in industrial catalytic applications, balancing activity with practical handling considerations.
Future Trends in Nickel Particle Research and Development
Research on nickel nanoparticles focuses on their enhanced catalytic, magnetic, and electrical properties, driven by their high surface area to volume ratio and quantum effects. Future trends emphasize sustainable synthesis methods and applications in energy storage, biomedicine, and environmental remediation, surpassing performance limits of nickel microparticles. Advancements in controlled particle size distribution and functionalization techniques will enable tailored properties for next-generation nanodevices and green technologies.
Nickel nanoparticle vs Nickel microparticle Infographic
 materialdif.com
materialdif.com