Sintering is a process that compacts and forms powdered metal materials into a solid mass by heating them below their melting point, enhancing material properties and shaping efficiency. Smelting involves extracting metal from its ore by heating and melting, separating metal from impurities through chemical reduction. Understanding the differences between sintering and smelting is essential for optimizing metal production and improving the quality of final metal products.
Table of Comparison
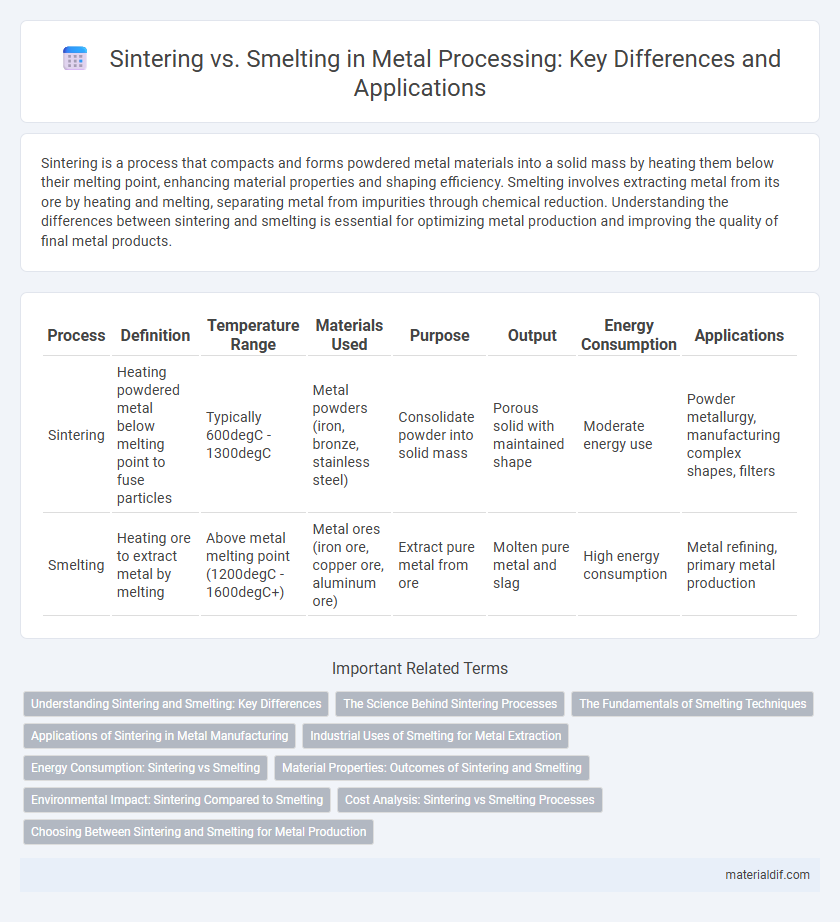
| Process | Definition | Temperature Range | Materials Used | Purpose | Output | Energy Consumption | Applications |
|---|---|---|---|---|---|---|---|
| Sintering | Heating powdered metal below melting point to fuse particles | Typically 600degC - 1300degC | Metal powders (iron, bronze, stainless steel) | Consolidate powder into solid mass | Porous solid with maintained shape | Moderate energy use | Powder metallurgy, manufacturing complex shapes, filters |
| Smelting | Heating ore to extract metal by melting | Above metal melting point (1200degC - 1600degC+) | Metal ores (iron ore, copper ore, aluminum ore) | Extract pure metal from ore | Molten pure metal and slag | High energy consumption | Metal refining, primary metal production |
Understanding Sintering and Smelting: Key Differences
Sintering involves heating powdered metal below its melting point to bond particles through diffusion, creating a solid mass without liquefaction. Smelting is a high-temperature process that melts ore to extract metal by separating impurities through chemical reduction. Understanding these key differences highlights sintering's role in powder metallurgy and smelting's function in primary metal extraction.
The Science Behind Sintering Processes
Sintering is a solid-state process that involves heating powdered metals below their melting point to enable particle bonding through diffusion, resulting in a dense and strong material. Unlike smelting, which melts ore to extract metals, sintering preserves the powder's solid form while enhancing its mechanical properties and microstructure. This process relies on atomic diffusion, surface energy reduction, and neck growth between particles to achieve desired metallurgical characteristics.
The Fundamentals of Smelting Techniques
Smelting techniques involve extracting metals from their ores by heating beyond the melting point to separate impurities, relying on chemical reduction processes to transform metal oxides into pure metals. This fundamental metallurgical process typically uses high temperatures and reducing agents such as carbon to facilitate the separation of metal from slag. Smelting is distinct from sintering, which compacts powdered materials without melting, emphasizing the crucial role of thermal and chemical reactions in metal purification and extraction.
Applications of Sintering in Metal Manufacturing
Sintering is extensively applied in powder metallurgy to produce complex metal parts with precise dimensions, enhancing material utilization and reducing waste compared to traditional casting. This process is ideal for manufacturing high-performance components such as gears, filters, and cutting tools by enabling controlled porosity and improved mechanical properties. Unlike smelting, sintering operates at temperatures below the melting point, preserving fine microstructures essential for aerospace, automotive, and medical implant applications.
Industrial Uses of Smelting for Metal Extraction
Smelting plays a crucial role in industrial metal extraction by efficiently separating metals from their ores using high-temperature chemical reduction processes. This technique is essential for producing large quantities of metals such as iron, copper, and aluminum, which are foundational for construction, automotive manufacturing, and electronics industries. Unlike sintering, which prepares ore materials for smelting, smelting directly yields pure metals ready for alloying and further processing in industrial applications.
Energy Consumption: Sintering vs Smelting
Sintering consumes less energy compared to smelting due to its lower temperature requirements, typically operating between 1200degC and 1400degC, whereas smelting requires temperatures above 1500degC to extract metals from ores. The reduced energy consumption in sintering enhances its efficiency in preparing raw materials for blast furnaces, leading to cost savings and lowered carbon emissions in metallurgical processes. Smelting's higher energy demand is attributed to the need for complete chemical reduction and separation of metals from impurities.
Material Properties: Outcomes of Sintering and Smelting
Sintering produces metal components with fine, uniform microstructures, enhancing mechanical strength, wear resistance, and dimensional accuracy due to controlled diffusion bonding without melting. Smelting results in molten metal that solidifies into coarse-grained structures, offering higher purity and improved ductility but often requiring further refining for specific mechanical properties. Sintered materials typically exhibit superior porosity control, beneficial for filters and porous parts, whereas smelted metals provide bulk material suitable for casting and forging applications.
Environmental Impact: Sintering Compared to Smelting
Sintering produces lower greenhouse gas emissions than smelting by operating at comparatively lower temperatures and reusing exhaust gases, reducing overall carbon footprint. Smelting releases significant amounts of sulfur dioxide and heavy metals into the atmosphere, contributing to air pollution and acid rain. Sintering also allows for partial recycling of particulate matter, minimizing solid waste generation compared to smelting's greater slag production.
Cost Analysis: Sintering vs Smelting Processes
Sintering offers lower operational costs compared to smelting due to reduced energy consumption and simpler equipment requirements, making it cost-effective for processing fine metal powders. Smelting involves higher expenses driven by intensive energy use and complex furnace maintenance, often justified only for high-purity metal extraction. In large-scale production, sintering reduces raw material waste and enhances yield, ultimately lowering overall processing costs relative to smelting.
Choosing Between Sintering and Smelting for Metal Production
Choosing between sintering and smelting for metal production depends on factors like the desired metal purity, energy consumption, and raw material form. Sintering, which agglomerates fine metal powders through heat below the melting point, offers energy efficiency and is ideal for preparing ores with impurities for further processing. Smelting involves melting ore at high temperatures to extract pure metal, suitable for metals requiring high purity but demands significantly higher energy input.
Sintering vs Smelting Infographic
 materialdif.com
materialdif.com