Lead-acid batteries offer higher surge currents and are more cost-effective for large-scale energy storage, making them ideal for automotive and backup power applications. Nickel-cadmium batteries provide superior performance in extreme temperatures and longer cycle life, which benefits portable electronics and aviation uses. Each battery type has distinct advantages based on energy density, cost, and environmental considerations.
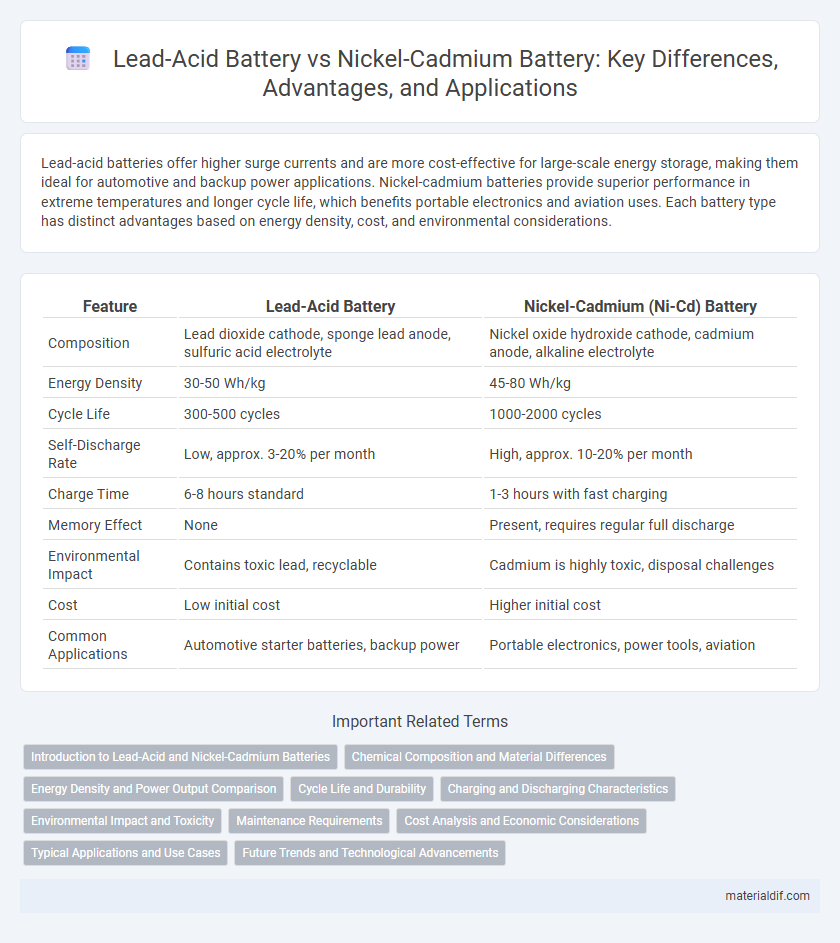
Table of Comparison
| Feature | Lead-Acid Battery | Nickel-Cadmium (Ni-Cd) Battery |
|---|---|---|
| Composition | Lead dioxide cathode, sponge lead anode, sulfuric acid electrolyte | Nickel oxide hydroxide cathode, cadmium anode, alkaline electrolyte |
| Energy Density | 30-50 Wh/kg | 45-80 Wh/kg |
| Cycle Life | 300-500 cycles | 1000-2000 cycles |
| Self-Discharge Rate | Low, approx. 3-20% per month | High, approx. 10-20% per month |
| Charge Time | 6-8 hours standard | 1-3 hours with fast charging |
| Memory Effect | None | Present, requires regular full discharge |
| Environmental Impact | Contains toxic lead, recyclable | Cadmium is highly toxic, disposal challenges |
| Cost | Low initial cost | Higher initial cost |
| Common Applications | Automotive starter batteries, backup power | Portable electronics, power tools, aviation |
Introduction to Lead-Acid and Nickel-Cadmium Batteries
Lead-acid batteries utilize lead dioxide and sponge lead plates submerged in sulfuric acid electrolyte, offering reliable energy storage with high surge currents suitable for automotive and backup power applications. Nickel-cadmium (Ni-Cd) batteries consist of nickel oxide hydroxide cathodes and cadmium anodes with an alkaline electrolyte, known for their durability, long cycle life, and ability to perform well under extreme temperatures. Both battery types differ significantly in energy density, environmental impact, and maintenance requirements, influencing their selection for various industrial and consumer uses.
Chemical Composition and Material Differences
Lead-acid batteries use lead dioxide (PbO2) and metallic lead (Pb) as electrodes submerged in sulfuric acid (H2SO4) electrolyte, offering high surge currents and cost-effectiveness. Nickel-cadmium (NiCd) batteries consist of nickel oxide hydroxide (NiOOH) cathodes, metallic cadmium (Cd) anodes, and potassium hydroxide (KOH) electrolyte, providing superior cycle life and better performance at low temperatures. The key material difference lies in the lead components versus cadmium usage, influencing toxicity, energy density, and rechargeability characteristics.
Energy Density and Power Output Comparison
Lead-acid batteries have a lower energy density, typically around 30-50 Wh/kg, compared to nickel-cadmium batteries which offer 45-80 Wh/kg, enabling Ni-Cd batteries to store more energy per unit weight. In terms of power output, nickel-cadmium batteries excel with higher discharge rates and better performance under high load conditions, making them suitable for power-intensive applications. Lead-acid batteries, while heavier and bulkier, still provide reliable power but are less efficient for high-drain devices due to their limited energy density and slower charge acceptance.
Cycle Life and Durability
Lead-acid batteries typically offer a cycle life of 300 to 500 cycles and are less durable under deep discharge conditions compared to nickel-cadmium batteries, which can withstand 1,000 to 1,500 cycles with superior resistance to overcharging and high temperatures. Nickel-cadmium batteries maintain performance consistency over prolonged usage due to their robust electrode structure and ability to tolerate more extreme environmental variations. The enhanced durability and longer cycle life of nickel-cadmium cells make them preferable for applications requiring frequent cycling and extended operational lifespan.
Charging and Discharging Characteristics
Lead-acid batteries exhibit slower charging rates but provide stable voltage output during discharge, making them suitable for applications requiring steady power delivery. Nickel-cadmium batteries support rapid charging and maintain consistent performance across a wide temperature range, with a lower risk of voltage depression during discharge cycles. The charge retention and cycle life of nickel-cadmium batteries outperform lead-acid batteries, particularly under frequent deep discharge conditions.
Environmental Impact and Toxicity
Lead-acid batteries contain lead, a highly toxic heavy metal that poses significant environmental hazards through soil and water contamination if improperly disposed of, whereas nickel-cadmium batteries contain cadmium, a carcinogenic and persistent toxic substance harmful to aquatic life. Both battery types require careful recycling to mitigate toxic metal leaching, but nickel-cadmium batteries are more challenging to recycle due to cadmium's high toxicity and strict disposal regulations. Lead-acid batteries have a well-established recycling infrastructure, reducing their overall environmental footprint compared to the more ecotoxic and less frequently recycled nickel-cadmium cells.
Maintenance Requirements
Lead-acid batteries require regular maintenance such as checking electrolyte levels and cleaning terminals to prevent corrosion, which ensures optimal performance and longevity. Nickel-cadmium batteries demand less upkeep, with minimal electrolyte monitoring and no corrosion issues, making them suitable for applications where low maintenance is critical. Both battery types benefit from periodic inspections, but lead-acid batteries generally possess higher maintenance needs due to their chemical composition and design.
Cost Analysis and Economic Considerations
Lead-acid batteries offer a lower upfront cost and are widely used in automotive and backup power applications due to their affordability and ease of recycling. Nickel-cadmium batteries, although more expensive initially, provide longer cycle life and better performance in extreme temperatures, which can lead to cost savings over time. Economic considerations must include maintenance costs, environmental disposal fees, and the total cost of ownership to determine the most viable battery choice for specific applications.
Typical Applications and Use Cases
Lead-acid batteries are commonly used in automotive starters, uninterruptible power supplies (UPS), and energy storage systems due to their cost-effectiveness and high surge current capability. Nickel-cadmium (Ni-Cd) batteries excel in portable power tools, aviation, and emergency lighting because of their long cycle life and reliable performance under extreme temperatures. Both battery types serve distinct roles based on application requirements such as energy density, durability, and operational environment.
Future Trends and Technological Advancements
Lead-acid batteries continue to see improvements in energy density and charge efficiency through advanced materials like carbon additives, enhancing their viability for stationary energy storage and automotive applications. Nickel-cadmium batteries benefit from innovations in electrode design and nanomaterials, boosting cycle life and performance in extreme temperatures, which supports their use in aerospace and medical devices. Emerging trends emphasize sustainability, with both battery types undergoing recycling technology advancements and integration into hybrid energy systems to meet future energy storage demands.
Lead-acid battery vs Nickel-cadmium battery Infographic
 materialdif.com
materialdif.com