Lead oxide and barium oxide are both metal oxides with distinct chemical properties and industrial applications. Lead oxide is commonly used in lead-acid batteries and glass manufacturing due to its high density and ability to improve thermal stability, whereas barium oxide finds its use in ceramics, optical glass, and as a catalyst in chemical reactions because of its high melting point and basicity. Understanding the differences in toxicity and environmental impact is crucial, as lead oxide poses significant health risks, while barium oxide, although hazardous, has different exposure concerns.
Table of Comparison
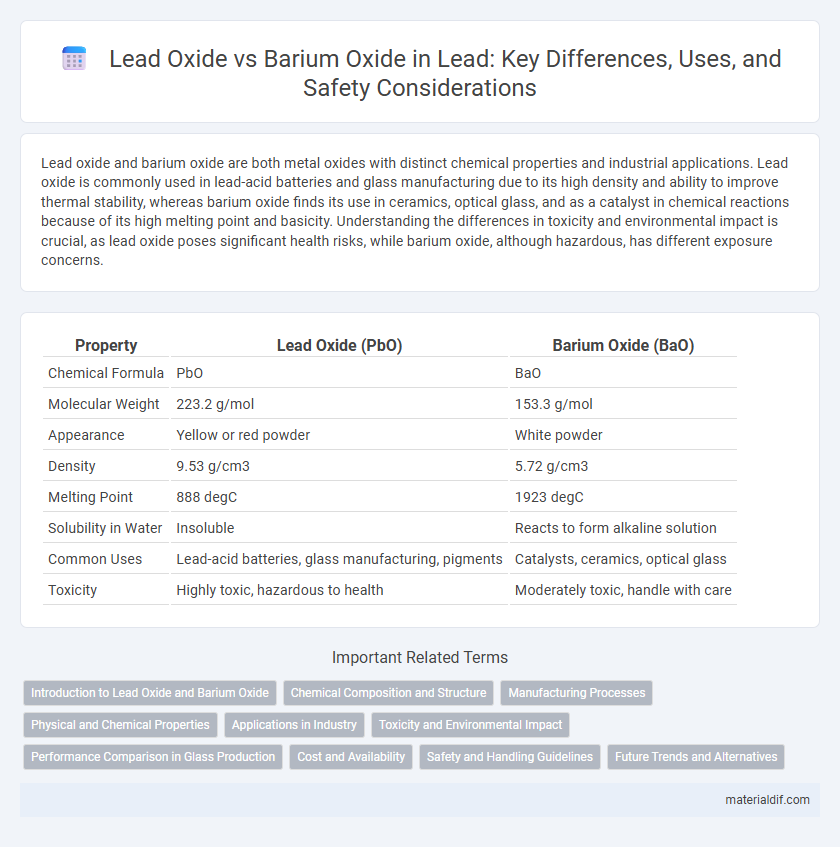
| Property | Lead Oxide (PbO) | Barium Oxide (BaO) |
|---|---|---|
| Chemical Formula | PbO | BaO |
| Molecular Weight | 223.2 g/mol | 153.3 g/mol |
| Appearance | Yellow or red powder | White powder |
| Density | 9.53 g/cm3 | 5.72 g/cm3 |
| Melting Point | 888 degC | 1923 degC |
| Solubility in Water | Insoluble | Reacts to form alkaline solution |
| Common Uses | Lead-acid batteries, glass manufacturing, pigments | Catalysts, ceramics, optical glass |
| Toxicity | Highly toxic, hazardous to health | Moderately toxic, handle with care |
Introduction to Lead Oxide and Barium Oxide
Lead oxide is an inorganic compound commonly used in batteries, ceramics, and glass production due to its semiconducting properties and high density. Barium oxide, a white hygroscopic solid, functions as a strong base and is integral in ceramics, glass manufacturing, and catalysts. Both oxides exhibit distinct chemical behaviors and applications influenced by their unique crystal structures and reactivity.
Chemical Composition and Structure
Lead oxide (PbO) consists of lead and oxygen atoms arranged in a tetragonal or orthorhombic crystal structure, depending on the polymorph, while barium oxide (BaO) features a simple cubic lattice formed by barium and oxygen ions. PbO exhibits a mixed ionic-covalent bonding character due to the Pb2+ cation's unique electronic configuration, whereas BaO's bonding is predominantly ionic with Ba2+ and O2- ions. The differing oxidation states and ionic radii of lead and barium contribute significantly to variations in their lattice parameters and chemical reactivity.
Manufacturing Processes
Lead oxide manufacturing primarily involves the oxidation of metallic lead through methods such as the Barton Pot process or the ball mill process, where lead is molten and exposed to air to form red or yellow lead oxide. Barium oxide production typically requires the calcination of barium carbonate at temperatures exceeding 1000degC in a kiln, decomposing it into barium oxide and carbon dioxide. Both processes emphasize high-temperature conditions but differ in raw materials and chemical reactions, influencing their industrial applications and purity levels.
Physical and Chemical Properties
Lead oxide (PbO) is a heavy, dense solid with a melting point of approximately 888degC and exhibits amphoteric behavior, reacting both as an acid and a base in chemical reactions. In contrast, barium oxide (BaO) is a white, alkaline solid with a higher melting point around 1923degC and primarily acts as a basic oxide, readily reacting with water to form barium hydroxide. The distinct physical states and reactivity profiles of PbO and BaO stem from their differing crystal structures and elemental properties, impacting their industrial applications and chemical handling.
Applications in Industry
Lead oxide is extensively used in the manufacturing of lead-acid batteries, glass, and ceramics due to its electrical conductivity and chemical stability. Barium oxide finds critical applications in the production of ceramics, glass, and as a catalyst in petroleum refining processes because of its high melting point and catalytic properties. Both oxides serve unique industrial purposes, with lead oxide predominating in energy storage solutions and barium oxide enhancing chemical processing and material durability.
Toxicity and Environmental Impact
Lead oxide exhibits high toxicity due to lead's neurotoxic effects, causing severe environmental and health hazards through soil and water contamination, bioaccumulation, and persistence in ecosystems. Barium oxide, while less toxic than lead oxide, still poses significant risks including respiratory irritation and potential water pollution incidents but degrades more readily in natural conditions. Regulatory frameworks strictly control lead oxide emissions and disposal due to its prolonged environmental persistence, whereas barium oxide's risks necessitate careful handling but generally offer a lower ecological threat.
Performance Comparison in Glass Production
Lead oxide offers superior refractive index enhancement and improves the brilliance and weight of glass compared to barium oxide, making it ideal for optical and decorative glass applications. Barium oxide, while providing good chemical durability and increased resistance to discoloration at high temperatures, generally results in lower density and less refractive enhancement than lead oxide. The choice between lead oxide and barium oxide in glass production depends on balancing optical performance with environmental and health considerations due to lead's toxicity.
Cost and Availability
Lead oxide is generally more expensive and less readily available than barium oxide due to stricter regulations on lead compounds and their environmental impact. Barium oxide benefits from abundant natural barium sources and lower production costs, making it a more cost-effective choice in industrial applications. Market demand and regional supply also influence the price and accessibility of these metal oxides.
Safety and Handling Guidelines
Lead oxide poses significant health risks due to its toxic lead content, requiring strict handling protocols including the use of personal protective equipment (PPE), proper ventilation, and measures to prevent inhalation or ingestion. Barium oxide, though also hazardous, primarily demands caution against skin and eye contact as it is highly alkaline and corrosive, necessitating gloves, eye protection, and immediate washing if exposed. Both oxides must be stored securely in labeled containers away from incompatible substances, with emergency procedures in place to manage spills and prevent environmental contamination.
Future Trends and Alternatives
Lead oxide is increasingly scrutinized due to its toxicity, prompting a shift toward safer alternatives like barium oxide in various industrial applications. Future trends emphasize the development of eco-friendly and sustainable oxides with improved electrical and thermal properties, favoring barium oxide for use in ceramics, glass, and electronics. Research advances in nanotechnology and green chemistry aim to replace lead oxide, reducing environmental impact while maintaining performance.
Lead Oxide vs Barium Oxide Infographic
 materialdif.com
materialdif.com