Lead and mercury are both heavy metals with significant toxicity risks, but lead primarily affects the nervous system and accumulates in bones, while mercury targets the brain and kidneys, often causing irreversible damage. Lead poisoning is common in industrial settings and old paints, whereas mercury exposure often results from contaminated seafood and industrial pollutants. Understanding their distinct toxicokinetics and sources is crucial for effective prevention and treatment strategies.
Table of Comparison
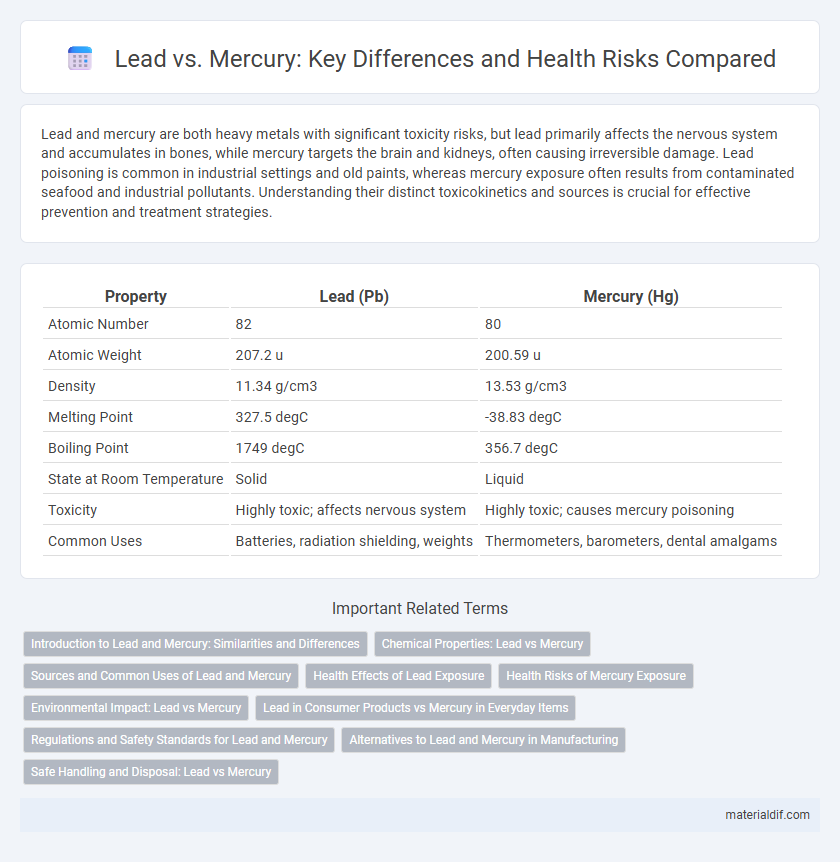
| Property | Lead (Pb) | Mercury (Hg) |
|---|---|---|
| Atomic Number | 82 | 80 |
| Atomic Weight | 207.2 u | 200.59 u |
| Density | 11.34 g/cm3 | 13.53 g/cm3 |
| Melting Point | 327.5 degC | -38.83 degC |
| Boiling Point | 1749 degC | 356.7 degC |
| State at Room Temperature | Solid | Liquid |
| Toxicity | Highly toxic; affects nervous system | Highly toxic; causes mercury poisoning |
| Common Uses | Batteries, radiation shielding, weights | Thermometers, barometers, dental amalgams |
Introduction to Lead and Mercury: Similarities and Differences
Lead and mercury are heavy metals with significant industrial and environmental impacts, both exhibiting high densities and toxicity. Lead is a malleable, bluish-gray metal primarily used in batteries, radiation shielding, and construction, whereas mercury is a liquid metal notable for its use in thermometers, barometers, and fluorescent lighting. Despite their similar toxicological risks, mercury's liquid state at room temperature contrasts with lead's solid form, influencing their applications and environmental behavior.
Chemical Properties: Lead vs Mercury
Lead and mercury differ significantly in their chemical properties; lead is a stable, soft metal with a high density of 11.34 g/cm3 and exhibits poor electrical conductivity, whereas mercury is a liquid metal at room temperature with a density of 13.56 g/cm3 and excellent conductivity. Lead's common oxidation states are +2 and +4, forming compounds like lead oxide (PbO), while mercury primarily exhibits +1 and +2 states, creating mercurous (Hg22+) and mercuric (Hg2+) ions. Chemical reactivity varies as lead resists oxidation in air due to a protective oxide layer, whereas mercury readily amalgamates with metals and evaporates, posing unique chemical handling considerations.
Sources and Common Uses of Lead and Mercury
Lead is primarily sourced from galena ore and commonly used in batteries, radiation shielding, and construction materials. Mercury is extracted from cinnabar ore and frequently found in thermometers, dental amalgams, and fluorescent lighting. Both metals have distinct industrial applications due to their unique physical and chemical properties.
Health Effects of Lead Exposure
Lead exposure causes severe neurological damage, developmental delays, and cognitive impairments, especially in children. Unlike mercury, lead accumulates in bones and tissues, causing chronic toxicity that affects the cardiovascular and renal systems. Prolonged lead poisoning can result in anemia, hypertension, and irreversible brain damage, highlighting the critical need for exposure reduction.
Health Risks of Mercury Exposure
Mercury exposure poses significant health risks including neurological damage, cognitive impairments, and kidney toxicity, often more severe than those associated with lead. Unlike lead, mercury can bioaccumulate in the food chain, particularly in fish, leading to higher human exposure through dietary intake. Acute and chronic mercury poisoning symptoms include tremors, memory loss, and impaired motor skills, demanding immediate medical attention to prevent long-term health consequences.
Environmental Impact: Lead vs Mercury
Lead contamination primarily affects soil and water, causing long-term toxicity that harms plants, animals, and human health through bioaccumulation. Mercury releases, especially from industrial sources, transform into methylmercury in aquatic systems, leading to highly toxic conditions that accumulate in fish and pose severe neurological risks to wildlife and humans. Both metals persist in the environment, but mercury's ability to bio-magnify makes its ecological and health impacts more acute in aquatic ecosystems.
Lead in Consumer Products vs Mercury in Everyday Items
Lead is commonly used in consumer products such as batteries, paints, and plumbing materials due to its durability and malleability. In contrast, mercury is found in everyday items like thermometers, fluorescent lamps, and dental amalgams, valued for its liquid state at room temperature. The toxicity of lead in household items raises concerns, whereas mercury's unique properties make it essential in precision instruments despite its hazardous nature.
Regulations and Safety Standards for Lead and Mercury
Lead and mercury are subject to strict regulations and safety standards due to their toxicological risks, with agencies like the EPA and OSHA setting exposure limits and handling protocols. Lead regulations focus on minimizing exposure in environments such as construction, manufacturing, and consumer products, enforcing limits like the EPA's lead content restrictions in paint and plumbing. Mercury safety standards regulate emissions from industrial processes and control mercury content in products through frameworks like the Minamata Convention on Mercury to prevent environmental contamination and human health hazards.
Alternatives to Lead and Mercury in Manufacturing
Alternatives to lead and mercury in manufacturing include tin, bismuth, and tungsten, which offer lower toxicity and comparable material properties for applications like soldering and battery production. Silicon-based materials and organic compounds are increasingly used in electronics and thermometers to replace harmful heavy metals. Advances in safer, sustainable materials contribute to reducing environmental and health risks associated with lead and mercury exposure.
Safe Handling and Disposal: Lead vs Mercury
Lead requires careful handling with protective gloves and adequate ventilation due to its neurotoxic effects, and disposal must follow hazardous waste regulations to prevent soil and water contamination. Mercury, being a volatile and highly toxic metal, demands specialized containment measures to avoid vapor inhalation, with disposal handled through certified hazardous waste facilities that prevent air and water pollution. Both metals pose significant environmental and health risks if mishandled, emphasizing strict adherence to safety protocols during handling and disposal.
Lead vs Mercury Infographic
 materialdif.com
materialdif.com