Lead and antimony are both heavy metals commonly used in industrial applications, but they differ significantly in properties and toxicity. Lead is softer and more malleable, making it suitable for batteries and shielding, while antimony enhances hardness and strength when alloyed, often used in flame retardants and semiconductors. Understanding the distinct chemical behaviors of lead versus antimony is crucial for safe handling and effective utilization in manufacturing processes.
Table of Comparison
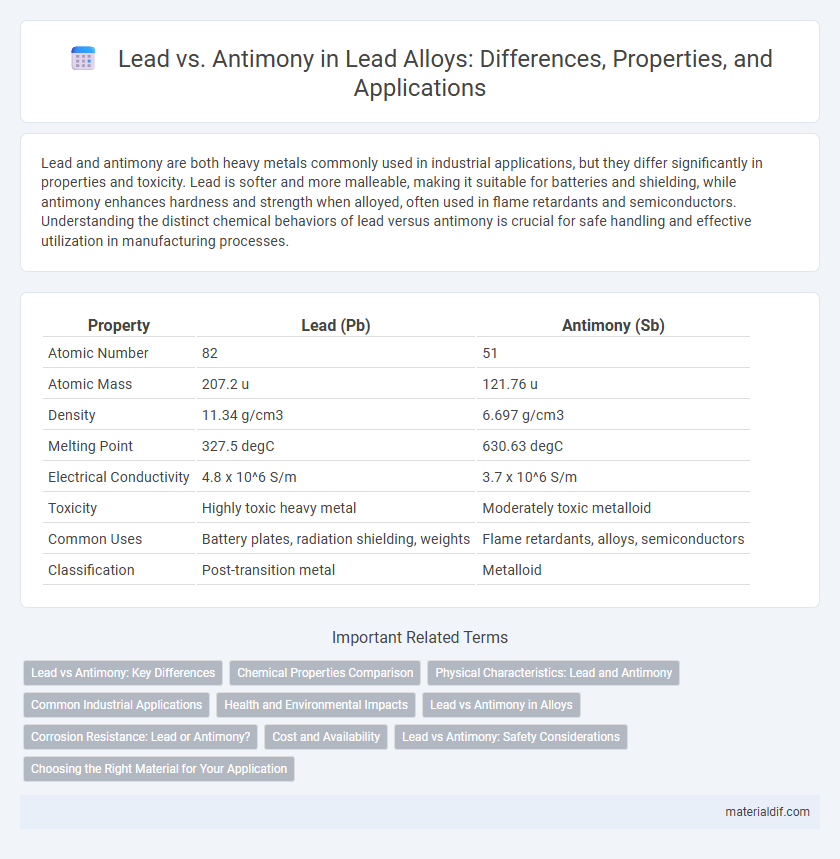
| Property | Lead (Pb) | Antimony (Sb) |
|---|---|---|
| Atomic Number | 82 | 51 |
| Atomic Mass | 207.2 u | 121.76 u |
| Density | 11.34 g/cm3 | 6.697 g/cm3 |
| Melting Point | 327.5 degC | 630.63 degC |
| Electrical Conductivity | 4.8 x 10^6 S/m | 3.7 x 10^6 S/m |
| Toxicity | Highly toxic heavy metal | Moderately toxic metalloid |
| Common Uses | Battery plates, radiation shielding, weights | Flame retardants, alloys, semiconductors |
| Classification | Post-transition metal | Metalloid |
Lead vs Antimony: Key Differences
Lead and antimony differ significantly in chemical properties and applications, with lead (Pb) being a heavy metal known for its high density and toxicity, while antimony (Sb) is a metalloid valued for its flame-retardant properties. Lead exhibits excellent malleability and corrosion resistance, commonly used in batteries and radiation shielding, whereas antimony enhances alloys, such as in lead-acid batteries and soldering materials, due to its hardness and brittleness. The environmental and health impacts also vary, as lead exposure poses severe neurological risks, contrasting with antimony's moderate toxicity and role in industrial flame retardants.
Chemical Properties Comparison
Lead exhibits a higher atomic number (82) compared to antimony (51), influencing its denser atomic structure and greater malleability. Chemically, lead is less reactive, forming stable +2 oxidation states, whereas antimony commonly exhibits +3 and +5 oxidation states, contributing to its versatility in compound formation. Lead's inertness makes it resistant to corrosion, contrasting with antimony's semi-metallic nature, which facilitates its use in flame retardants and alloys.
Physical Characteristics: Lead and Antimony
Lead exhibits a dense, malleable, and soft metallic structure with a bluish-gray color, while antimony is harder, brittle, and silver-white with a slight bluish tinge. Lead has a higher density of approximately 11.34 g/cm3 compared to antimony's 6.68 g/cm3, influencing their applications in weight-sensitive and protective materials. Antimony's crystalline structure gives it greater rigidity, contrasting with lead's more ductile nature, which enhances lead's use in plumbing and radiation shielding.
Common Industrial Applications
Lead is widely used in batteries, radiation shielding, and cable sheathing due to its high density and corrosion resistance, while antimony is primarily employed as a flame retardant in plastics, textiles, and as an alloying element in lead-acid batteries to improve hardness. Both metals find critical roles in electronics and construction; lead's toxicity concerns drive regulatory restrictions, whereas antimony's flame-retardant properties enhance safety in consumer goods. Industrial sectors rely on lead for energy storage and protection from radiation, whereas antimony's contributions ensure material durability and fire resistance.
Health and Environmental Impacts
Lead exposure is linked to severe neurological damage, particularly in children, causing cognitive deficits and behavioral disorders, and it persists in the environment due to its non-biodegradable nature. Antimony, while less neurotoxic than lead, poses risks of respiratory irritation, skin disorders, and potential carcinogenic effects, with environmental concerns stemming from its accumulation in soil and water. Both metals require stringent regulation and remediation to mitigate long-term health hazards and ecological contamination.
Lead vs Antimony in Alloys
Lead and antimony are both commonly used alloying elements, with lead primarily enhancing machinability and corrosion resistance, while antimony increases hardness and mechanical strength. In alloys such as lead-acid batteries, antimony is added to lead to improve creep resistance and structural stability under high temperatures. The balance between lead and antimony content influences properties like tensile strength, wear resistance, and casting quality in automotive and industrial components.
Corrosion Resistance: Lead or Antimony?
Lead exhibits superior corrosion resistance in acidic and saline environments compared to antimony, making it more suitable for applications exposed to harsh conditions. Antimony, while harder and more wear-resistant, tends to oxidize and corrode faster when exposed to moisture and acids. Therefore, lead is typically preferred in industries such as battery manufacturing and marine environments where corrosion resistance is critical.
Cost and Availability
Lead is generally more cost-effective and widely available compared to antimony, making it a preferred choice in industries requiring bulk material use. Antimony's limited natural reserves and complex extraction processes contribute to its higher price and relative scarcity. Market demand fluctuations further impact antimony's availability, driving costs above those of commonly sourced lead.
Lead vs Antimony: Safety Considerations
Lead and antimony both pose significant health hazards, with lead primarily causing neurological damage and antimony linked to respiratory and skin irritation. Lead exposure often occurs through ingestion or inhalation, requiring strict workplace controls and personal protective equipment to minimize risk. Antimony safety measures emphasize proper ventilation and protective gear to prevent inhalation of dust or fumes, as chronic exposure can lead to lung and heart problems.
Choosing the Right Material for Your Application
Lead offers superior corrosion resistance and excellent malleability, making it ideal for applications requiring durability and easy shaping, such as batteries and radiation shielding. Antimony enhances hardness and mechanical strength when alloyed with lead, benefiting industries needing wear resistance like ammunition and cable sheathing. Selecting between lead and antimony depends on whether flexibility or hardness is prioritized in your specific application.
Lead vs Antimony Infographic
 materialdif.com
materialdif.com