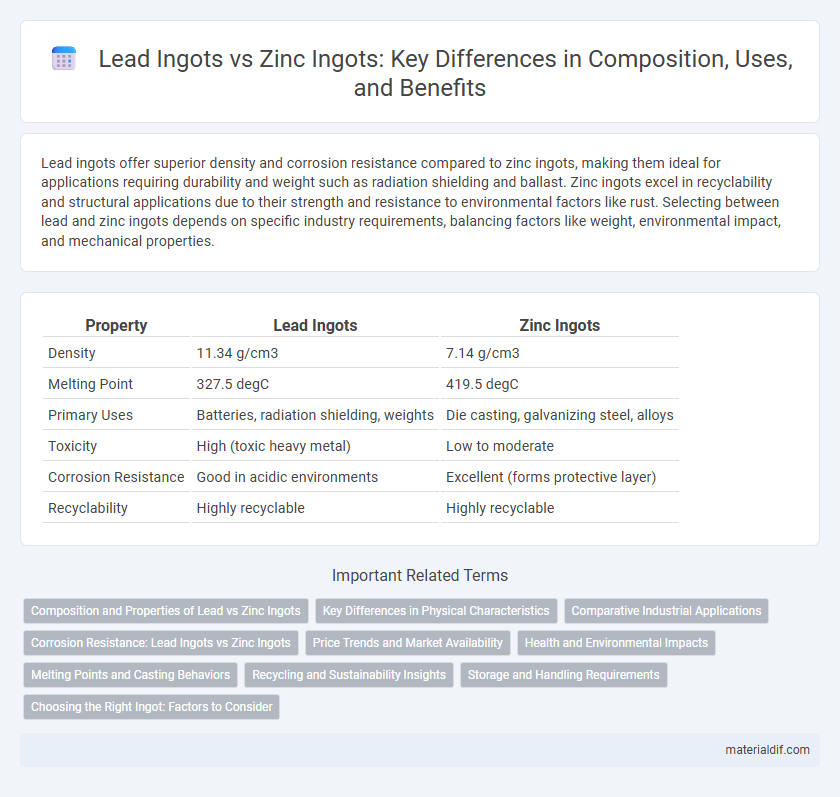
Lead ingots offer superior density and corrosion resistance compared to zinc ingots, making them ideal for applications requiring durability and weight such as radiation shielding and ballast. Zinc ingots excel in recyclability and structural applications due to their strength and resistance to environmental factors like rust. Selecting between lead and zinc ingots depends on specific industry requirements, balancing factors like weight, environmental impact, and mechanical properties.
Table of Comparison
| Property | Lead Ingots | Zinc Ingots |
|---|---|---|
| Density | 11.34 g/cm3 | 7.14 g/cm3 |
| Melting Point | 327.5 degC | 419.5 degC |
| Primary Uses | Batteries, radiation shielding, weights | Die casting, galvanizing steel, alloys |
| Toxicity | High (toxic heavy metal) | Low to moderate |
| Corrosion Resistance | Good in acidic environments | Excellent (forms protective layer) |
| Recyclability | Highly recyclable | Highly recyclable |
Composition and Properties of Lead vs Zinc Ingots
Lead ingots primarily consist of about 99.85% pure lead, known for their high density of 11.34 g/cm3, low melting point around 327.5degC, and excellent corrosion resistance, making them ideal for radiation shielding and ballast applications. Zinc ingots, composed of approximately 99.99% pure zinc, exhibit a lower density of 7.14 g/cm3 and a higher melting point of 419.5degC, with superior strength and resistance to atmospheric corrosion, suitable for galvanizing steel and die-casting processes. The fundamental differences in composition influence their physical properties, where lead's softness and malleability contrast with zinc's hardness and brittleness.
Key Differences in Physical Characteristics
Lead ingots exhibit high density and softness, with a bluish-gray color and low melting point around 327.5degC, making them malleable and easy to cast. Zinc ingots are lighter with moderate density, possess a silver-blue sheen, and have a higher melting point of approximately 419.5degC, resulting in greater hardness and brittleness compared to lead. These physical differences significantly influence the choice of ingot material for applications requiring specific weight, strength, or melting properties.
Comparative Industrial Applications
Lead ingots are predominantly used in battery manufacturing, radiation shielding, and construction due to their high density and corrosion resistance. Zinc ingots find extensive application in galvanization, die-casting alloys, and brass production, primarily because of their excellent corrosion protection and malleability. Industrial sectors select lead or zinc ingots based on specific requirements such as strength, weight, and environmental resistance, optimizing material performance for specialized uses.
Corrosion Resistance: Lead Ingots vs Zinc Ingots
Lead ingots exhibit superior corrosion resistance compared to zinc ingots due to lead's natural inertness and ability to form a protective oxide layer, making it ideal for applications in harsh environments. Zinc ingots, while less corrosion-resistant, provide sacrificial protection through galvanization, effectively preventing rust on steel surfaces. Understanding the differing corrosion properties of lead and zinc ingots is crucial for selecting materials in construction, marine, and industrial settings.
Price Trends and Market Availability
Lead ingots generally exhibit more stable price trends due to consistent demand in battery manufacturing and construction industries, while zinc ingots experience higher price volatility influenced by galvanization and alloy production fluctuations. Market availability of lead ingots remains relatively steady because of established recycling streams and steady industrial use, whereas zinc ingots face occasional supply constraints linked to mining outputs and seasonal demand shifts. Recent market data shows lead prices maintaining moderate growth, whereas zinc prices have seen sharper short-term spikes reflecting tighter supply chains.
Health and Environmental Impacts
Lead ingots pose significant health risks due to lead's neurotoxicity and potential to cause serious ailments such as lead poisoning, especially in children and pregnant women. Zinc ingots, by contrast, present fewer direct human health hazards but can still impact the environment through mining and processing activities that contribute to soil and water contamination. Proper handling, recycling, and disposal protocols are critical for both materials to mitigate environmental pollution and protect public health.
Melting Points and Casting Behaviors
Lead ingots have a melting point of approximately 327.5degC, allowing for easy melting and casting with minimal energy consumption, while zinc ingots melt at around 419.5degC, requiring higher temperatures for processing. The lower melting point of lead ingots facilitates smoother casting behaviors, producing finer grain structures and fewer casting defects compared to zinc ingots. Zinc ingots tend to exhibit faster solidification rates, which can lead to increased porosity and challenges in achieving uniform castings.
Recycling and Sustainability Insights
Lead ingots exhibit high recyclability rates due to their low melting point and efficient recovery processes, making them a cornerstone in sustainable metal reuse. Zinc ingots also offer significant recycling potential, with advanced purification techniques enhancing the quality of recycled material and reducing environmental impact. Both metals contribute to circular economy practices by minimizing raw material extraction and lowering carbon footprints through closed-loop recycling systems.
Storage and Handling Requirements
Lead ingots require storage in dry, well-ventilated areas to prevent oxidation and contamination, with adequate containment to avoid heavy metal exposure hazards. Zinc ingots demand storage in temperature-controlled environments to minimize corrosion and maintain structural integrity, coupled with careful handling to prevent surface damage and dust generation. Both metals necessitate the use of personal protective equipment (PPE) during handling to ensure worker safety and compliance with regulatory standards.
Choosing the Right Ingot: Factors to Consider
Selecting between lead ingots and zinc ingots requires evaluating factors such as density, corrosion resistance, and melting point; lead ingots offer high density and excellent radiation shielding, while zinc ingots provide better corrosion resistance and are lighter. Consider the application environment, as lead's toxicity demands careful handling and disposal, whereas zinc is more environmentally friendly and suited for galvanization. Cost efficiency and material compatibility with existing systems also influence choosing the most appropriate ingot for manufacturing or construction needs.
Lead Ingots vs Zinc Ingots Infographic
 materialdif.com
materialdif.com