Lead glass offers higher refractive index and brilliance, making it popular for decorative items and optical lenses. Borosilicate glass provides superior thermal resistance and chemical durability, ideal for laboratory equipment and cookware. Choosing between lead glass and borosilicate glass depends on the application's need for clarity versus heat resistance.
Table of Comparison
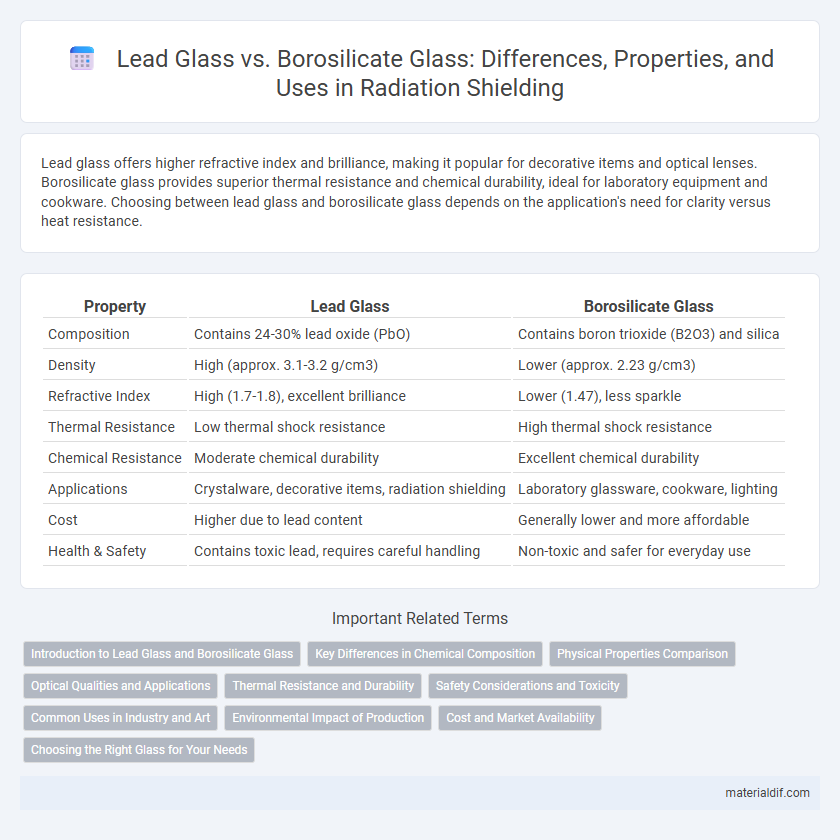
| Property | Lead Glass | Borosilicate Glass |
|---|---|---|
| Composition | Contains 24-30% lead oxide (PbO) | Contains boron trioxide (B2O3) and silica |
| Density | High (approx. 3.1-3.2 g/cm3) | Lower (approx. 2.23 g/cm3) |
| Refractive Index | High (1.7-1.8), excellent brilliance | Lower (1.47), less sparkle |
| Thermal Resistance | Low thermal shock resistance | High thermal shock resistance |
| Chemical Resistance | Moderate chemical durability | Excellent chemical durability |
| Applications | Crystalware, decorative items, radiation shielding | Laboratory glassware, cookware, lighting |
| Cost | Higher due to lead content | Generally lower and more affordable |
| Health & Safety | Contains toxic lead, requires careful handling | Non-toxic and safer for everyday use |
Introduction to Lead Glass and Borosilicate Glass
Lead glass, also known as lead crystal, contains a significant percentage of lead oxide, enhancing its refractive index and brilliance, making it ideal for decorative and optical applications. Borosilicate glass is composed primarily of silica and boron trioxide, offering exceptional thermal resistance and chemical durability, widely used in laboratory and cookware items. The key distinction between lead glass and borosilicate glass lies in their chemical composition and resulting physical properties, influencing their specific industrial and consumer uses.
Key Differences in Chemical Composition
Lead glass contains a significant amount of lead oxide, typically between 18% to 40%, which enhances its refractive index and brilliance, whereas borosilicate glass primarily consists of silica and boron trioxide, providing exceptional thermal and chemical resistance. The presence of lead oxide in lead glass increases its density and lowers its melting point compared to borosilicate glass, which features a higher melting point due to its borosilicate network. These compositional differences directly impact their physical properties, with lead glass favored for optical clarity and decorative uses, while borosilicate glass is preferred in scientific and laboratory applications for durability and heat resistance.
Physical Properties Comparison
Lead glass exhibits higher density and refractive index compared to borosilicate glass, resulting in enhanced brilliance and clarity. Borosilicate glass offers superior thermal resistance and lower thermal expansion, making it more suitable for high-temperature applications. The hardness of lead glass is generally lower, while borosilicate glass provides greater mechanical strength and durability.
Optical Qualities and Applications
Lead glass offers superior brilliance and clarity due to its high refractive index, making it ideal for decorative items and optical lenses where light dispersion is critical. Borosilicate glass excels in thermal and chemical resistance with moderate optical clarity, preferred in laboratory equipment and cookware requiring durability under temperature changes. Both materials serve distinct optical and practical applications based on their unique composition and performance characteristics.
Thermal Resistance and Durability
Lead glass exhibits lower thermal resistance compared to borosilicate glass, making it less suitable for high-temperature applications. Borosilicate glass offers exceptional durability and can withstand rapid temperature changes up to approximately 450degC without cracking. Its thermal shock resistance surpasses that of lead glass, ensuring longevity in demanding environments.
Safety Considerations and Toxicity
Lead glass contains significant amounts of lead oxide, which can pose safety risks through lead leaching, especially when used with acidic or hot substances, potentially causing toxicity. Borosilicate glass, composed primarily of silica and boron oxide, offers superior chemical resistance and thermal stability without the health hazards linked to lead exposure. Choosing borosilicate glass minimizes toxicological concerns and enhances safety in applications such as laboratory glassware and food containers.
Common Uses in Industry and Art
Lead glass is widely used in high-end decorative art, optical lenses, and radiation shielding due to its brilliance, high refractive index, and density. Borosilicate glass, valued for its thermal resistance and chemical durability, is preferred in laboratory equipment, pharmaceutical containers, and industrial lighting. Both materials serve distinct roles in industry and art, with lead glass favored for aesthetic and optical applications, while borosilicate glass excels in functional and heat-resistant uses.
Environmental Impact of Production
Lead glass production involves the use of heavy metals, which can result in higher environmental toxicity and challenges in waste management compared to borosilicate glass. Borosilicate glass manufacturing typically requires lower melting temperatures, leading to reduced energy consumption and a smaller carbon footprint. The recyclability of borosilicate glass is generally more favorable, contributing to a lesser overall environmental impact than lead glass.
Cost and Market Availability
Lead glass generally costs more than borosilicate glass due to its higher density and specialized manufacturing processes, making it less accessible in mass markets. Borosilicate glass is widely available and economically produced, favored for its thermal resistance and durability in scientific and everyday applications. Market availability of lead glass remains limited primarily to decorative and radiation shielding uses, whereas borosilicate glass dominates industries requiring cost-effective, high-performance glassware.
Choosing the Right Glass for Your Needs
Lead glass offers superior clarity and brilliance due to its high refractive index, making it ideal for decorative items and fine glassware. Borosilicate glass excels in thermal resistance and durability, suitable for laboratory equipment and cookware exposed to rapid temperature changes. Selecting the right glass depends on whether optical qualities or heat resistance is your primary requirement.
Lead Glass vs Borosilicate Glass Infographic
 materialdif.com
materialdif.com