Galena and Cerussite are two lead-containing minerals with distinct properties that impact their industrial and environmental significance. Galena, a lead sulfide, is the primary ore of lead due to its high lead content and ease of extraction, while Cerussite, a lead carbonate, forms through the oxidation of Galena and is valued for its lead purity but is less abundant. Understanding the differences in their chemical composition, formation processes, and economic uses is essential for effective lead ore processing and environmental management.
Table of Comparison
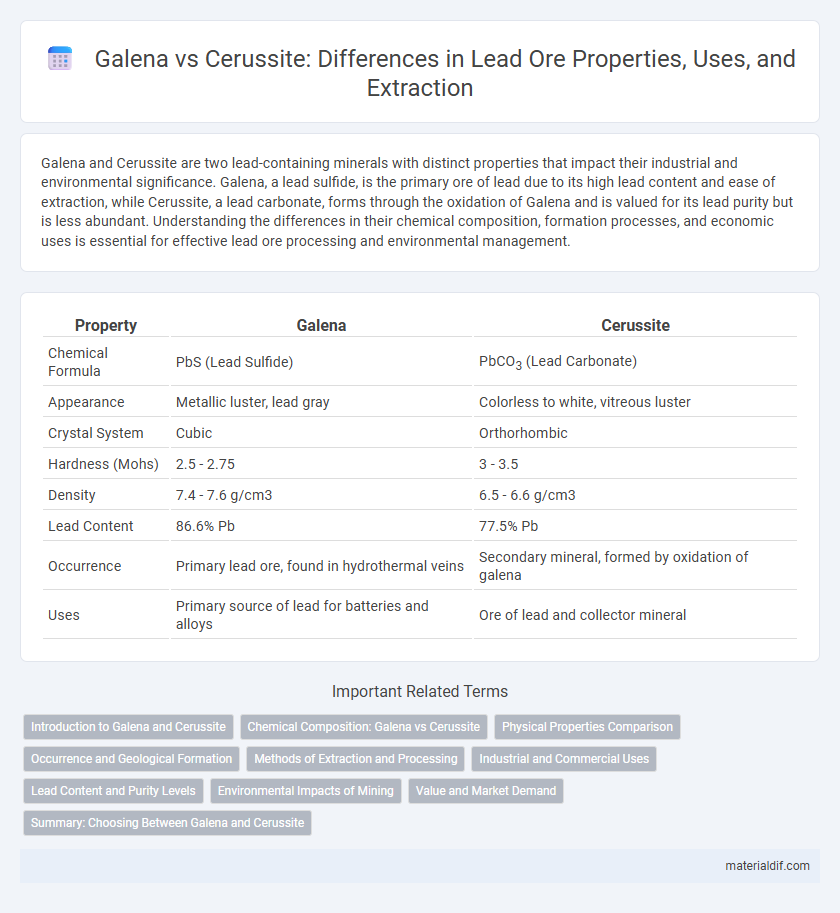
| Property | Galena | Cerussite |
|---|---|---|
| Chemical Formula | PbS (Lead Sulfide) | PbCO3 (Lead Carbonate) |
| Appearance | Metallic luster, lead gray | Colorless to white, vitreous luster |
| Crystal System | Cubic | Orthorhombic |
| Hardness (Mohs) | 2.5 - 2.75 | 3 - 3.5 |
| Density | 7.4 - 7.6 g/cm3 | 6.5 - 6.6 g/cm3 |
| Lead Content | 86.6% Pb | 77.5% Pb |
| Occurrence | Primary lead ore, found in hydrothermal veins | Secondary mineral, formed by oxidation of galena |
| Uses | Primary source of lead for batteries and alloys | Ore of lead and collector mineral |
Introduction to Galena and Cerussite
Galena, a primary ore of lead, is characterized by its metallic luster and cubic crystal structure, making it a major source of lead extraction worldwide. Cerussite, a secondary lead carbonate mineral, forms through the weathering of galena and is valued for its high lead content and unique crystal formations. Both minerals play crucial roles in the lead industry, with galena serving as the principal lead ore and cerussite contributing to lead recycling and refining processes.
Chemical Composition: Galena vs Cerussite
Galena (PbS) is a lead sulfide mineral composed primarily of lead and sulfur, making it the most important ore for lead extraction due to its high lead content and metallic luster. Cerussite (PbCO3) is a lead carbonate mineral containing lead, carbon, and oxygen, often forming through the weathering and oxidation of galena deposits. The chemical difference between Galena's sulfide composition and Cerussite's carbonate composition influences their physical properties, stability, and methods of lead extraction.
Physical Properties Comparison
Galena presents a metallic luster with a high density of approximately 7.4 to 7.6 g/cm3, while Cerussite exhibits a vitreous to adamantine luster and a lower density ranging from 6.5 to 6.6 g/cm3. Galena crystallizes in the cubic system forming distinct cubic or octahedral crystals, whereas Cerussite crystallizes in the orthorhombic system, often showing twinned or reticulated crystal habits. Both minerals differ in hardness, with Galena rating around 2.5 on the Mohs scale and Cerussite being slightly harder at 3 to 3.5, influencing their respective uses and handling procedures.
Occurrence and Geological Formation
Galena primarily occurs in hydrothermal veins, often associated with minerals like sphalerite, chalcopyrite, and fluorite, forming in low to medium temperature environments within sedimentary and metamorphic rocks. Cerussite generally forms as a secondary mineral through the oxidation of galena in the upper zones of lead ore deposits, particularly in arid climates where lead sulfides undergo weathering. The geological formation of galena is typically linked to primary sulfide mineralization, whereas cerussite represents a secondary carbonate mineral formed in supergene enrichment zones.
Methods of Extraction and Processing
Galena is primarily extracted through froth flotation, which efficiently separates lead sulfide from gangue minerals, while cerussite is commonly processed using gravity separation and leaching techniques due to its carbonate nature. Galena's processing includes roasting to convert lead sulfide into lead oxide before smelting, whereas cerussite undergoes acid leaching to dissolve lead carbonate into lead salts. These differing methods reflect the distinct mineralogy of lead ores, impacting recovery rates and environmental management.
Industrial and Commercial Uses
Galena, primarily composed of lead sulfide, is the most important ore for extracting lead used in batteries, ammunition, and radiation shielding due to its high lead content and ease of processing. Cerussite, a lead carbonate mineral, is less abundant and mainly utilized in specialized applications such as pigment production and certain chemical processes. Industrially, galena's dominance ensures its preference in large-scale lead extraction, while cerussite serves niche commercial roles where its chemical properties are advantageous.
Lead Content and Purity Levels
Galena (PbS) contains approximately 86.6% lead by weight, making it the primary ore for lead extraction with high purity levels. Cerussite (PbCO3) has a slightly lower lead content, around 77.5%, but often yields a high-grade product due to its carbonate composition. Industrial processing typically favors galena for lead production due to its richer lead concentration and ease of refining into pure lead metal.
Environmental Impacts of Mining
Galena mining releases significant amounts of lead and sulfur dioxide, contributing to soil and water contamination and acid rain, which pose severe environmental and health risks. Cerussite extraction generates substantial lead dust and tailings that contaminate nearby ecosystems, affecting aquatic life and vegetation through heavy metal accumulation. Effective environmental management strategies are essential to mitigate toxic emissions and prevent long-term ecological damage from both Galena and Cerussite mining operations.
Value and Market Demand
Galena, the primary ore of lead, holds greater market demand due to its higher lead content and accessibility, making it more valuable for industrial applications. Cerussite, although richer in lead concentration, is less abundant and more challenging to process, limiting its commercial value despite its purity. Market trends favor Galena for large-scale lead extraction, while Cerussite remains a niche source valued for specialty uses and mineral collections.
Summary: Choosing Between Galena and Cerussite
Galena and cerussite are two primary lead ores with distinct characteristics and applications. Galena, rich in lead sulfide, offers higher lead content and is more commonly mined for industrial lead production due to its ease of extraction and processing. Cerussite, a lead carbonate mineral, is often valued for its potential as a secondary lead source and in environmental remediation, but it requires more complex processing techniques.
Galena vs Cerussite Infographic
 materialdif.com
materialdif.com