Lead and solder serve different purposes in electronics, with lead primarily used as a core component in solder alloys rather than a standalone material. Solder, typically composed of a mixture of tin and lead or lead-free alternatives, melts at a lower temperature to join electronic components securely without damaging them. The choice between lead-based and lead-free solder affects the environmental impact, with lead-free options becoming more popular due to health and safety regulations.
Table of Comparison
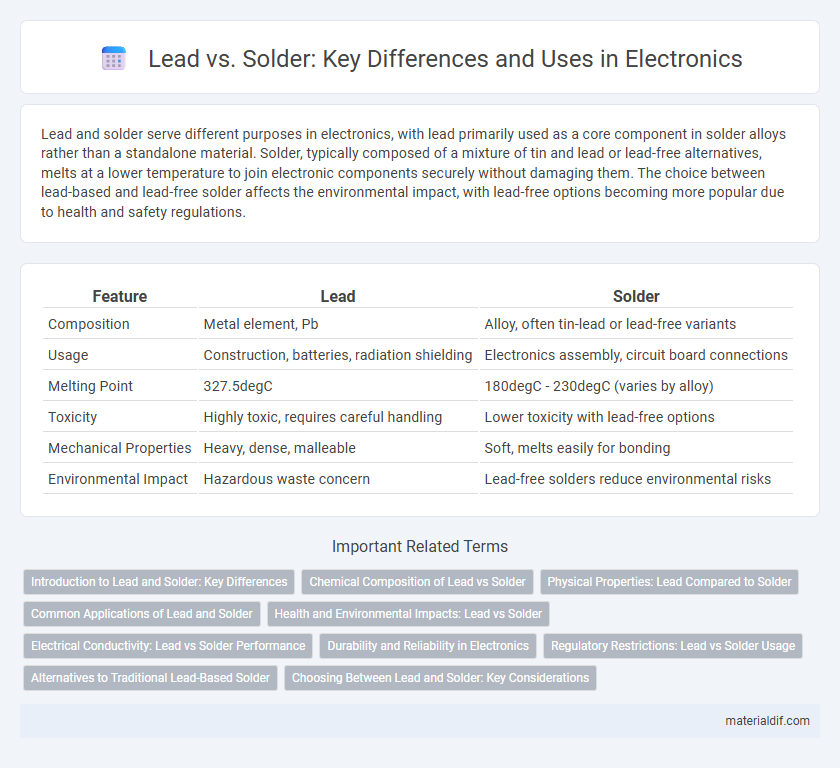
| Feature | Lead | Solder |
|---|---|---|
| Composition | Metal element, Pb | Alloy, often tin-lead or lead-free variants |
| Usage | Construction, batteries, radiation shielding | Electronics assembly, circuit board connections |
| Melting Point | 327.5degC | 180degC - 230degC (varies by alloy) |
| Toxicity | Highly toxic, requires careful handling | Lower toxicity with lead-free options |
| Mechanical Properties | Heavy, dense, malleable | Soft, melts easily for bonding |
| Environmental Impact | Hazardous waste concern | Lead-free solders reduce environmental risks |
Introduction to Lead and Solder: Key Differences
Lead is a dense metal with a low melting point commonly used in batteries, shielding, and historically in pipes, while solder is a fusible alloy primarily composed of tin and lead or lead-free alternatives designed to join metal parts. The key difference lies in their function: lead serves as a raw material with various industrial applications, whereas solder is specifically formulated for creating reliable electrical and mechanical bonds. Understanding their composition and purposes highlights critical distinctions in safety, usage, and regulatory compliance within manufacturing and electronics.
Chemical Composition of Lead vs Solder
Lead is a chemical element with the symbol Pb and atomic number 82, characterized by its dense, malleable properties and high resistance to corrosion. Solder, primarily composed of a tin-lead alloy, varies in composition but typically contains 60-70% tin and 30-40% lead for optimal melting point and mechanical strength. Modern solders increasingly use lead-free alternatives made from tin, silver, and copper to meet environmental regulations and improve joint reliability.
Physical Properties: Lead Compared to Solder
Lead exhibits a higher density of approximately 11.34 g/cm3 compared to typical solder alloys, which range around 8.4 g/cm3 to 8.7 g/cm3, making lead significantly heavier. The melting point of pure lead is about 327.5degC, whereas common solder alloys, such as 60/40 tin-lead solder, melt around 183degC, enabling easier melting and bonding in electronic applications. Lead's lower thermal conductivity and higher malleability contribute to its distinct handling characteristics versus solder, which is designed to form strong, electrically conductive joints.
Common Applications of Lead and Solder
Lead is commonly used in batteries, radiation shielding, and ammunition due to its high density and corrosion resistance, while solder is primarily utilized in electronics for joining metal components with its excellent electrical conductivity and low melting point. Lead-based solder historically dominated circuit board assembly, but modern applications often favor lead-free alternatives in solder to meet environmental regulations. Both materials play distinct roles in manufacturing and maintenance, with lead serving structural and protective functions and solder ensuring reliable electrical connections.
Health and Environmental Impacts: Lead vs Solder
Lead poses significant health risks due to its toxicity, causing neurological damage and developmental issues upon prolonged exposure. Traditional lead-based solder contributes to environmental contamination through lead leaching in soil and water, whereas lead-free solder alternatives reduce these hazards but may introduce other metals with different ecological impacts. Choosing lead-free solder improves workplace safety and aligns with global regulations aiming to minimize lead pollution and protect human health.
Electrical Conductivity: Lead vs Solder Performance
Lead exhibits lower electrical conductivity compared to most solder alloys, which typically contain tin, silver, or copper to enhance conductivity and mechanical strength. Solder's improved electrical conductivity ensures more efficient current flow in electronic connections, reducing resistance and heat generation. Choosing solder over pure lead improves circuit reliability and performance in electronic assemblies.
Durability and Reliability in Electronics
Lead offers superior durability and reliability in electronics due to its excellent mechanical strength and resistance to thermal fatigue. Solder, especially lead-free variants, tends to have lower flexibility, increasing the risk of crack formation in high-stress environments. Electronic components using lead-based connections typically demonstrate longer lifespans and enhanced performance stability under thermal cycling conditions.
Regulatory Restrictions: Lead vs Solder Usage
Regulatory restrictions significantly limit lead usage in electronics due to its toxicity, especially under RoHS and REACH directives that enforce maximum allowable concentrations. Solder alternatives, like lead-free alloys composed of tin, silver, and copper, comply with these regulations and reduce environmental and health risks. Manufacturers increasingly adopt lead-free solder to ensure product safety, regulatory compliance, and market access in regions with strict hazardous substance controls.
Alternatives to Traditional Lead-Based Solder
Traditional lead-based solder poses health and environmental risks, prompting a shift toward alternatives such as lead-free solder alloys composed of tin, silver, and copper. These lead-free options offer improved safety and comply with RoHS (Restriction of Hazardous Substances) regulations, widely adopted in electronics manufacturing. Emerging materials like conductive adhesives and nano-soldering techniques also provide innovative, eco-friendly solutions for electronic component assembly.
Choosing Between Lead and Solder: Key Considerations
Choosing between lead and solder involves evaluating toxicity, melting points, and application requirements. Lead, a heavy metal with high toxicity, offers durability but raises health concerns, while solder, typically a tin-based alloy with lower melting points, ensures safer and easier joining of electronic components. Assessing environmental impact, regulatory compliance, and mechanical strength is crucial for optimal material selection in manufacturing and repair processes.
Lead vs Solder Infographic
 materialdif.com
materialdif.com