Lead and zinc differ significantly in their chemical properties and environmental impact, with lead being a toxic heavy metal that poses serious health risks, while zinc is an essential trace element vital for biological functions. Lead exposure can cause neurological damage and contamination of soil and water, prompting strict regulations and efforts to reduce its use. In contrast, zinc is commonly used in alloys, galvanization, and dietary supplements, emphasizing its crucial role in industrial applications and human health.
Table of Comparison
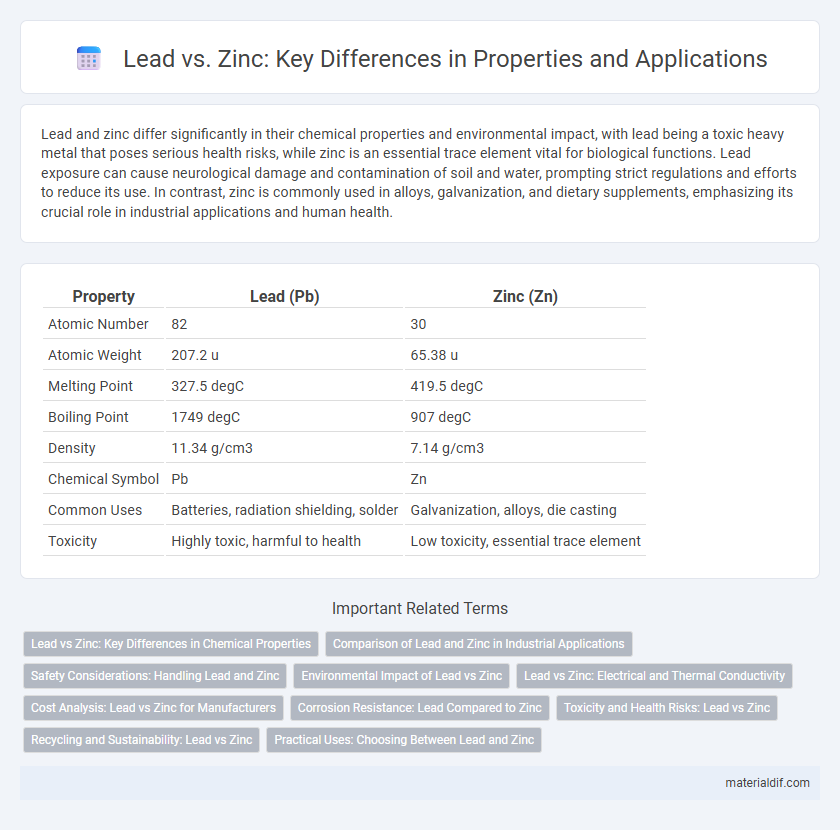
| Property | Lead (Pb) | Zinc (Zn) |
|---|---|---|
| Atomic Number | 82 | 30 |
| Atomic Weight | 207.2 u | 65.38 u |
| Melting Point | 327.5 degC | 419.5 degC |
| Boiling Point | 1749 degC | 907 degC |
| Density | 11.34 g/cm3 | 7.14 g/cm3 |
| Chemical Symbol | Pb | Zn |
| Common Uses | Batteries, radiation shielding, solder | Galvanization, alloys, die casting |
| Toxicity | Highly toxic, harmful to health | Low toxicity, essential trace element |
Lead vs Zinc: Key Differences in Chemical Properties
Lead and zinc differ significantly in chemical properties; lead has an atomic number of 82 with a higher atomic mass of 207.2 u compared to zinc's atomic number 30 and atomic mass 65.38 u. Lead exhibits lower reactivity, forms stable +2 and +4 oxidation states, and is highly resistant to corrosion, while zinc primarily shows a +2 oxidation state and acts as a sacrificial anode due to its higher electrochemical reactivity.
Comparison of Lead and Zinc in Industrial Applications
Lead and zinc serve distinct roles in industrial applications, with lead prized for its high density and excellent corrosion resistance, making it ideal for radiation shielding and batteries. Zinc, known for its galvanizing properties, is extensively used in corrosion protection of steel and in alloy production such as brass. While lead poses environmental and health risks due to toxicity, zinc offers a safer alternative with applications in construction, automotive, and electronics industries.
Safety Considerations: Handling Lead and Zinc
Handling lead requires strict safety measures due to its toxic properties, including using proper personal protective equipment (PPE) such as gloves and respirators to prevent ingestion or inhalation of lead dust or fumes. Zinc, while less toxic, still demands caution to avoid respiratory irritation from zinc oxide fumes, especially during welding or smelting processes, necessitating adequate ventilation and protective gear. Both metals require safe storage practices to minimize environmental contamination and human exposure risks.
Environmental Impact of Lead vs Zinc
Lead poses significant environmental risks due to its high toxicity, persistence in soil and water, and potential to bioaccumulate in ecosystems, causing harm to wildlife and human health. Zinc, although essential in small amounts and less toxic, can still cause environmental damage at elevated concentrations by disrupting aquatic life and soil microbiota. Proper management and remediation strategies are crucial for mitigating the environmental impact of both metals, with lead requiring more stringent controls due to its greater hazardous effects.
Lead vs Zinc: Electrical and Thermal Conductivity
Lead exhibits significantly lower electrical and thermal conductivity compared to zinc, making zinc a preferred choice for applications requiring efficient electricity and heat transfer. Zinc's electrical conductivity is approximately 16.6 million S/m, whereas lead's electrical conductivity is about 4.8 million S/m, highlighting zinc's superior performance. Furthermore, zinc also has higher thermal conductivity, around 116 W/m*K, in contrast to lead's low thermal conductivity of approximately 35 W/m*K.
Cost Analysis: Lead vs Zinc for Manufacturers
Lead offers a lower raw material cost compared to zinc, making it a more budget-friendly option for manufacturers focused on minimizing expenses. Zinc's higher price point is often justified by its superior corrosion resistance and environmental benefits, which can reduce long-term maintenance costs. Manufacturers must weigh initial cost savings of lead against potential lifecycle advantages and regulatory compliance costs associated with zinc usage.
Corrosion Resistance: Lead Compared to Zinc
Lead exhibits superior corrosion resistance compared to zinc due to its dense, stable oxide layer that effectively protects the metal from environmental degradation. Zinc corrodes more rapidly in acidic or saline conditions, forming zinc carbonate, which offers less protection than lead's oxide barrier. This makes lead a preferred choice for applications requiring long-term exposure to harsh environments, such as roofing and chemical storage tanks.
Toxicity and Health Risks: Lead vs Zinc
Lead exhibits significantly higher toxicity compared to zinc, posing severe health risks such as neurological damage, cognitive impairments, and cardiovascular problems, especially in children. Zinc, while essential in trace amounts for bodily functions, can become harmful only in excessive doses, typically causing gastrointestinal distress rather than chronic toxicity. Chronic lead exposure has been linked to irreversible health effects, whereas zinc toxicity is generally acute and reversible upon reducing intake.
Recycling and Sustainability: Lead vs Zinc
Lead and zinc recycling processes differ significantly in terms of environmental impact and sustainability; lead recycling is highly efficient and reduces hazardous waste due to its widespread use in batteries and industrial applications. Zinc recycling, while also efficient, offers added benefits by reducing the need for energy-intensive mining and decreases soil contamination through scrap metal recovery. Both metals contribute to circular economies, but lead's higher toxicity necessitates more stringent handling and recycling protocols to ensure environmental safety.
Practical Uses: Choosing Between Lead and Zinc
Lead is primarily used in batteries, radiation shielding, and weights due to its high density and malleability, while zinc is favored for galvanizing steel, die-casting, and alloy production because of its corrosion resistance and moderate strength. When choosing between lead and zinc, consider the application's environmental impact, with zinc being more eco-friendly and safer for human contact. Practical use often depends on required durability, toxicity concerns, and specific mechanical properties tailored to industrial or consumer needs.
Lead vs Zinc Infographic
 materialdif.com
materialdif.com