Lead and cadmium are both toxic heavy metals with significant health risks, but lead is primarily known for its impact on the nervous system, especially in children, causing cognitive impairments and developmental delays. Cadmium exposure mainly affects the kidneys and can lead to bone demineralization, increasing the risk of fractures and osteoporosis. Controlling exposure to these metals is crucial since both accumulate in the body and pose long-term health hazards.
Table of Comparison
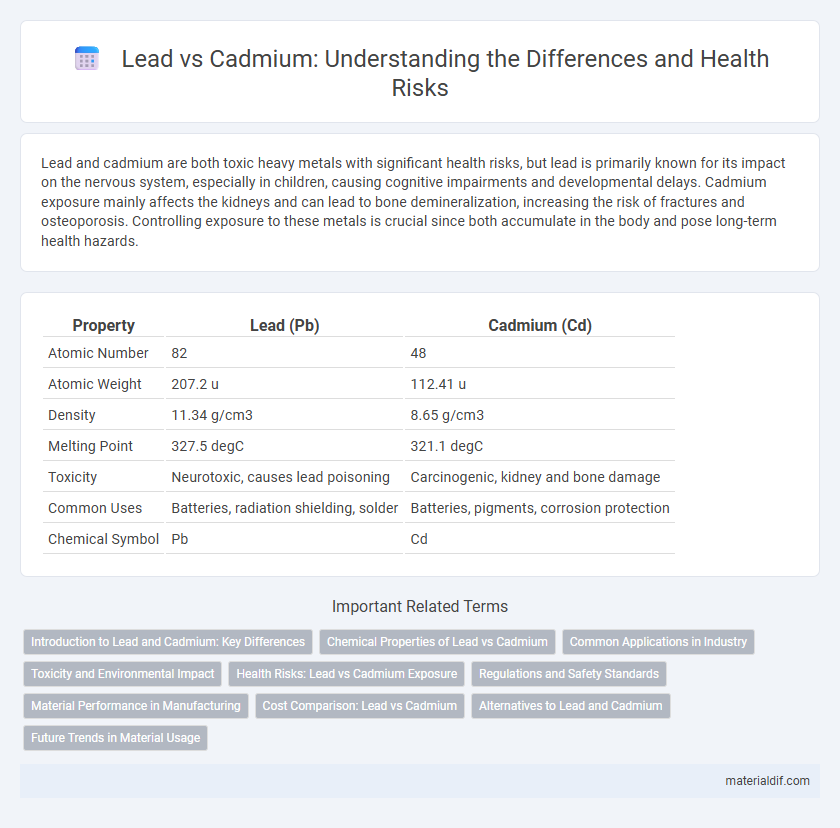
| Property | Lead (Pb) | Cadmium (Cd) |
|---|---|---|
| Atomic Number | 82 | 48 |
| Atomic Weight | 207.2 u | 112.41 u |
| Density | 11.34 g/cm3 | 8.65 g/cm3 |
| Melting Point | 327.5 degC | 321.1 degC |
| Toxicity | Neurotoxic, causes lead poisoning | Carcinogenic, kidney and bone damage |
| Common Uses | Batteries, radiation shielding, solder | Batteries, pigments, corrosion protection |
| Chemical Symbol | Pb | Cd |
Introduction to Lead and Cadmium: Key Differences
Lead and cadmium are heavy metals commonly found in industrial applications but differ significantly in toxicity and environmental impact. Lead is primarily used in batteries and construction materials, while cadmium is often found in rechargeable batteries and pigments. Understanding their chemical properties and health risks is essential for proper handling and regulatory compliance.
Chemical Properties of Lead vs Cadmium
Lead (Pb) has a higher atomic number of 82 and an atomic mass of 207.2 g/mol compared to cadmium (Cd), which has an atomic number of 48 and an atomic mass of 112.4 g/mol. Lead exhibits a lower electronegativity of 2.33 and forms more stable +2 oxidation states, whereas cadmium primarily exists in the +2 oxidation state but has higher electronegativity at 1.69. Both metals exhibit metallic bonding and are prone to oxidation, but lead's higher density (11.34 g/cm3) and melting point (327.5degC) contrast with cadmium's lower density (8.65 g/cm3) and melting point (321.1degC).
Common Applications in Industry
Lead is extensively used in the battery industry, particularly in lead-acid batteries for vehicles and backup power systems, due to its excellent energy storage and low cost. Cadmium finds common applications in rechargeable nickel-cadmium (NiCd) batteries and as a corrosion-resistant plating material in the electronics and aerospace industries. Both metals have specialized industrial roles, with lead favored for heavy-duty energy storage and cadmium preferred for durable, high-performance coatings and batteries.
Toxicity and Environmental Impact
Lead exhibits high toxicity, causing neurological damage and developmental disorders in humans, while cadmium primarily affects kidney function and bone density. Both metals persist in the environment, with lead accumulating in soil and water, and cadmium contaminating agricultural products through plant uptake. Environmental regulations aim to limit their emissions due to their bioaccumulative properties and long-term ecological harm.
Health Risks: Lead vs Cadmium Exposure
Lead exposure results in neurotoxicity, cognitive impairment, and cardiovascular issues, significantly impacting children's brain development. Cadmium exposure primarily causes kidney damage, bone demineralization, and respiratory problems due to its accumulation in the lungs. Both heavy metals pose serious health risks through prolonged exposure, necessitating stringent controls to minimize human contact.
Regulations and Safety Standards
Lead and cadmium are both regulated heavy metals with strict safety standards due to their toxicity, but lead faces more comprehensive global restrictions, such as the U.S. EPA's Lead and Copper Rule and the European Union's REACH regulations. Cadmium is primarily controlled under the RoHS Directive and the Basel Convention, targeting its use in electronics and waste management to prevent environmental contamination. Regulatory agencies emphasize minimizing human exposure through limits in consumer products, workplace safety standards, and environmental discharge controls.
Material Performance in Manufacturing
Lead exhibits superior malleability and corrosion resistance compared to cadmium, making it more suitable for high-durability manufacturing applications. Cadmium offers enhanced conductivity and lighter weight but falls short in mechanical strength and long-term stability under stress. Material performance in manufacturing favors lead for components requiring longevity and resistance to environmental degradation.
Cost Comparison: Lead vs Cadmium
Lead is significantly more cost-effective than cadmium, with prices typically lower due to its abundance and easier extraction processes. Cadmium, being rarer and primarily obtained as a byproduct of zinc refining, commands higher market prices, increasing overall material costs. This cost disparity makes lead the preferred metal for applications demanding affordability without specialized metal properties.
Alternatives to Lead and Cadmium
Alternatives to lead and cadmium include non-toxic metals such as tin, bismuth, and zinc, which provide safer options in applications like soldering and batteries. Advanced materials like lithium-ion and sodium-ion batteries reduce reliance on heavy metals, enhancing environmental sustainability. Researchers prioritize developing biodegradable and low-toxicity compounds to replace lead and cadmium in electronics and pigments.
Future Trends in Material Usage
Lead is increasingly being replaced by cadmium-free alternatives in battery and electronic applications due to stricter environmental regulations and health concerns. Emerging technologies favor cadmium-free compounds like lithium-ion and nickel-metal hydride batteries, which offer higher energy density and lower toxicity. Future material usage trends emphasize sustainable and non-toxic elements, reducing reliance on hazardous substances like lead and cadmium in manufacturing processes.
Lead vs Cadmium Infographic
 materialdif.com
materialdif.com