Lead oxide exhibits higher reactivity and greater toxicity compared to lead carbonate, making it more hazardous in industrial applications. Lead carbonate is less soluble and more stable, often preferred in pigments and coatings due to its lower environmental impact. Understanding their chemical properties is crucial for safer handling and regulatory compliance in manufacturing processes.
Table of Comparison
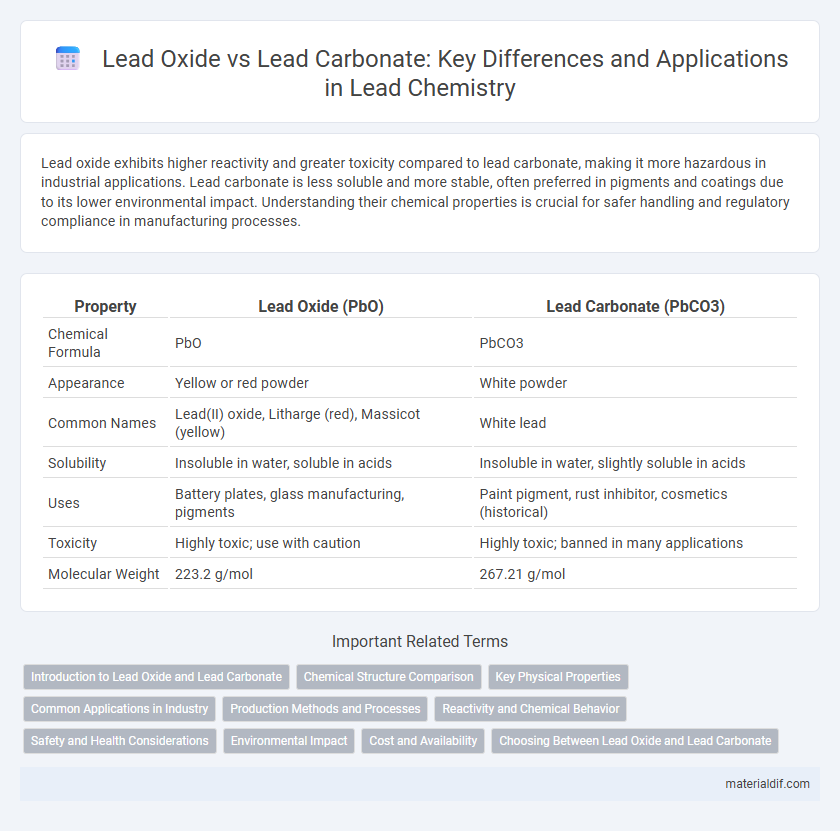
| Property | Lead Oxide (PbO) | Lead Carbonate (PbCO3) |
|---|---|---|
| Chemical Formula | PbO | PbCO3 |
| Appearance | Yellow or red powder | White powder |
| Common Names | Lead(II) oxide, Litharge (red), Massicot (yellow) | White lead |
| Solubility | Insoluble in water, soluble in acids | Insoluble in water, slightly soluble in acids |
| Uses | Battery plates, glass manufacturing, pigments | Paint pigment, rust inhibitor, cosmetics (historical) |
| Toxicity | Highly toxic; use with caution | Highly toxic; banned in many applications |
| Molecular Weight | 223.2 g/mol | 267.21 g/mol |
Introduction to Lead Oxide and Lead Carbonate
Lead oxide and lead carbonate are two common lead compounds with distinct chemical properties and industrial applications. Lead oxide, typically appearing as a reddish or yellowish powder, is widely used in the manufacture of batteries, ceramics, and glass due to its high reactivity and oxide content. Lead carbonate, also known as white lead, is a basic lead salt commonly used as a pigment in paints and has significant historical importance despite its toxicity and environmental concerns.
Chemical Structure Comparison
Lead oxide (PbO) consists of lead ions bonded to oxygen ions forming a simple binary compound with a stable lattice structure, whereas lead carbonate (PbCO3) features lead ions bonded to carbonate groups (CO3) incorporating carbon and oxygen atoms in a polyatomic ion arrangement. The chemical structure of lead oxide is characterized by a direct lead-oxygen ionic bond, resulting in a crystalline form such as litharge or massicot. Lead carbonate's structure is more complex, involving coordination between lead ions and the trigonal planar carbonate anion, creating a more intricate ionic network.
Key Physical Properties
Lead oxide (PbO) appears as a yellow or red powder with a melting point of approximately 888degC, exhibiting amphoteric behavior and moderate solubility in acids. Lead carbonate (PbCO3), known as white lead ore or cerussite, is a white crystalline solid with a melting point around 315degC and limited solubility in water but reacts readily with acids. Both compounds are dense, with lead carbonate having a density of about 6.5 g/cm3 and lead oxide slightly lower, influencing their industrial applications in pigments and stabilizers.
Common Applications in Industry
Lead oxide serves as a critical component in the manufacture of lead-acid batteries, glass, and ceramics due to its excellent electrical conductivity and chemical stability. Lead carbonate is primarily utilized as a white pigment in paints and as a corrosion inhibitor in coatings, offering durability and protection. Both compounds play vital roles in industrial processes, with lead oxide favored in energy storage and electronics, while lead carbonate excels in protective and decorative applications.
Production Methods and Processes
Lead oxide is primarily produced through the oxidation of metallic lead in controlled air conditions at high temperatures, often utilizing the Barton pot or ball mill process. Lead carbonate, also known as cerussite, is typically synthesized by reacting lead nitrate or lead acetate with sodium carbonate in aqueous solutions, forming a precipitate through a controlled chemical precipitation method. The production of lead oxide involves thermal oxidation, while lead carbonate relies on chemical precipitation, influencing their purity and particle morphology.
Reactivity and Chemical Behavior
Lead oxide exhibits higher reactivity than lead carbonate due to its ability to readily participate in redox reactions, acting as both an oxidizing and reducing agent. Lead carbonate is more stable and primarily undergoes decomposition under heat, releasing carbon dioxide while forming lead oxide. The chemical behavior of lead oxide allows it to interact with acids and bases, forming various lead salts, whereas lead carbonate's interactions are limited by its lower solubility and reactivity.
Safety and Health Considerations
Lead oxide and lead carbonate pose significant health risks due to their toxic lead content, with lead oxide generally being more hazardous because of its higher solubility and potential for inhalation exposure. Both compounds can cause lead poisoning, affecting the nervous system, kidneys, and reproductive health, necessitating strict handling precautions, including personal protective equipment and adequate ventilation. Regulatory limits for occupational exposure and proper disposal methods are critical to minimize environmental contamination and human health hazards.
Environmental Impact
Lead oxide exhibits higher toxicity and poses greater environmental hazards due to its solubility and potential to release lead ions into soil and water, leading to contamination and bioaccumulation in ecosystems. Lead carbonate, while also toxic, is less soluble and tends to persist longer in sediments, increasing the risk of prolonged environmental exposure and gradual lead release. Both compounds contribute significantly to lead pollution, but their environmental behaviors differ, influencing remediation strategies and ecological risk assessments.
Cost and Availability
Lead oxide is generally more cost-effective and widely available than lead carbonate due to its simpler production process and higher demand across industries such as battery manufacturing and pigments. Lead carbonate tends to be more expensive because of its specialized applications, including in certain protective coatings and as a precursor in chemical syntheses, which limits large-scale production. Market availability of lead oxide is stronger, supported by established supply chains and consistent industrial consumption.
Choosing Between Lead Oxide and Lead Carbonate
Lead oxide offers higher chemical reactivity and is commonly used in lead-acid batteries and glass production, providing superior electrical conductivity and heat resistance. Lead carbonate, valued for its stability and lower toxicity, is preferred in pigments and rust-resistant coatings due to its non-corrosive nature. Selecting between lead oxide and lead carbonate depends on application requirements, balancing factors like reactivity, toxicity, and environmental impact.
Lead oxide vs Lead carbonate Infographic
 materialdif.com
materialdif.com