Lead anodes offer superior corrosion resistance and stable voltage output, making them ideal for heavy-duty industrial applications. Graphite anodes provide excellent conductivity and cost-effectiveness but are more susceptible to wear and require frequent replacement. Choosing between lead and graphite anodes depends on balancing durability needs with budget constraints in electrochemical systems.
Table of Comparison
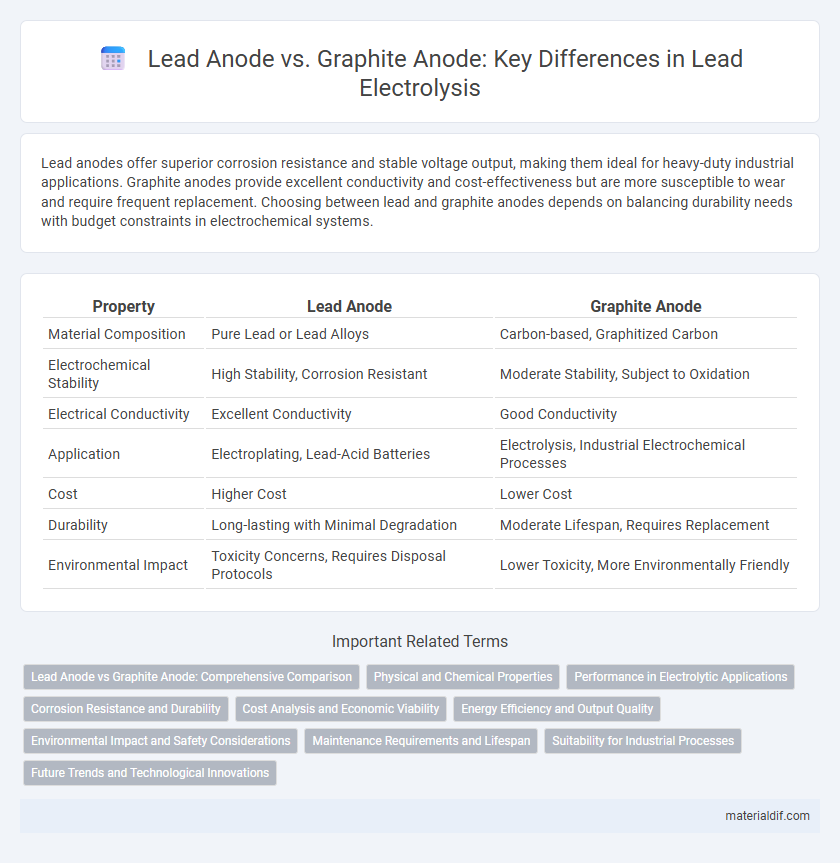
| Property | Lead Anode | Graphite Anode |
|---|---|---|
| Material Composition | Pure Lead or Lead Alloys | Carbon-based, Graphitized Carbon |
| Electrochemical Stability | High Stability, Corrosion Resistant | Moderate Stability, Subject to Oxidation |
| Electrical Conductivity | Excellent Conductivity | Good Conductivity |
| Application | Electroplating, Lead-Acid Batteries | Electrolysis, Industrial Electrochemical Processes |
| Cost | Higher Cost | Lower Cost |
| Durability | Long-lasting with Minimal Degradation | Moderate Lifespan, Requires Replacement |
| Environmental Impact | Toxicity Concerns, Requires Disposal Protocols | Lower Toxicity, More Environmentally Friendly |
Lead Anode vs Graphite Anode: Comprehensive Comparison
Lead anodes exhibit superior corrosion resistance and longer lifespan compared to graphite anodes, making them ideal for harsh industrial environments. Graphite anodes offer higher electrical conductivity and are more cost-effective, but they tend to degrade faster under heavy electrochemical stress. The choice between lead and graphite anodes depends on balancing durability, conductivity, and application-specific requirements in electrochemical processes.
Physical and Chemical Properties
Lead anodes exhibit high density and excellent corrosion resistance, making them ideal for applications requiring long-term durability in acidic environments. Graphite anodes feature lower density but superior electrical conductivity and good chemical inertness, enabling efficient current distribution and reduced energy loss. Both materials show distinct electrochemical stability ranges, with lead performing better under oxidative conditions and graphite excelling in alkaline media.
Performance in Electrolytic Applications
Lead anodes exhibit superior corrosion resistance and electrical conductivity in electrolytic applications, ensuring longer operational life compared to graphite anodes. Graphite anodes offer lower electrical efficiency and higher maintenance due to their susceptibility to erosion and consumption during electrolysis. Optimizing electrolytic performance often involves selecting lead anodes for stability and durability in harsh chemical environments.
Corrosion Resistance and Durability
Lead anodes exhibit superior corrosion resistance due to their self-healing oxide layer, making them highly durable in harsh electrochemical environments. Graphite anodes, while cost-effective and lightweight, are more prone to corrosion and surface degradation, reducing their lifespan in industrial applications. The enhanced durability of lead anodes contributes to longer maintenance intervals and improved overall system efficiency.
Cost Analysis and Economic Viability
Lead anodes exhibit lower initial costs compared to graphite anodes, making them economically viable for large-scale industrial applications. The durability and higher conductivity of lead anodes reduce maintenance and energy expenses over time, enhancing cost efficiency in continuous operations. Conversely, graphite anodes, while more expensive upfront, offer longer lifespan and resistance to corrosion, which can offset costs in long-term projects requiring minimal replacement.
Energy Efficiency and Output Quality
Lead anodes exhibit higher electrical conductivity, resulting in enhanced energy efficiency during electrochemical processes compared to graphite anodes. The output quality using lead anodes often features more consistent deposition and lower contamination levels, which optimizes product purity. Graphite anodes, while more cost-effective, generally require higher energy input and can contribute to uneven output quality due to carbon erosion and variable surface properties.
Environmental Impact and Safety Considerations
Lead anodes present higher environmental risks due to lead toxicity, posing significant challenges in disposal and potential soil and water contamination. Graphite anodes offer a safer alternative with lower environmental hazard, as graphite is non-toxic and more easily recyclable, reducing long-term ecological damage. Safety considerations further favor graphite anodes, as they do not release harmful fumes during operation, minimizing workplace health risks compared to lead anodes.
Maintenance Requirements and Lifespan
Lead anodes exhibit lower maintenance requirements due to their superior corrosion resistance and stable electrochemical performance, resulting in longer service life often exceeding 10 years in typical industrial applications. Graphite anodes, while initially cost-effective, require more frequent inspection and replacement within 3 to 5 years due to their susceptibility to abrasive wear and chemical degradation. Choosing lead anodes enhances operational efficiency by reducing downtime and maintenance costs associated with anode replacement and system contamination.
Suitability for Industrial Processes
Lead anodes exhibit superior chemical stability and corrosion resistance, making them ideal for harsh industrial electrochemical processes such as electroplating and wastewater treatment. Graphite anodes provide excellent electrical conductivity and are preferred in applications requiring high oxygen evolution potential, like chlor-alkali production. Choosing between lead and graphite anodes depends on the specific industrial environment, chemical compatibility, and process efficiency requirements.
Future Trends and Technological Innovations
Future trends in lead anode technology emphasize enhanced corrosion resistance and improved energy efficiency, driven by advancements in alloy composition and surface treatment techniques. Graphite anodes are experiencing innovations such as nanostructured coatings and increased conductivity materials, targeting longer lifespan and superior electrochemical performance. Integration of smart monitoring systems and sustainable manufacturing methods is poised to significantly impact the development and application of both lead and graphite anodes in industrial electrolysis processes.
Lead Anode vs Graphite Anode Infographic
 materialdif.com
materialdif.com