Lead and tin are both metals commonly used in soldering, but their properties differ significantly, impacting their suitability for various applications. Lead offers excellent malleability and low melting points, making it ideal for traditional soldering tasks, while tin provides a non-toxic alternative with better corrosion resistance and electrical conductivity. Modern electronics increasingly favor tin over lead due to environmental regulations and health concerns associated with lead exposure.
Table of Comparison
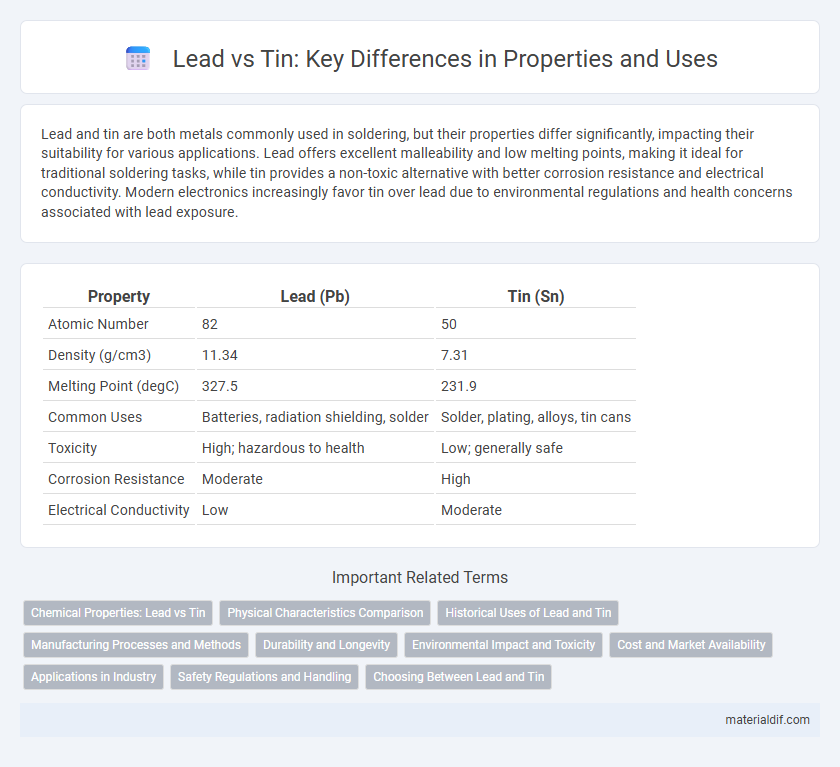
| Property | Lead (Pb) | Tin (Sn) |
|---|---|---|
| Atomic Number | 82 | 50 |
| Density (g/cm3) | 11.34 | 7.31 |
| Melting Point (degC) | 327.5 | 231.9 |
| Common Uses | Batteries, radiation shielding, solder | Solder, plating, alloys, tin cans |
| Toxicity | High; hazardous to health | Low; generally safe |
| Corrosion Resistance | Moderate | High |
| Electrical Conductivity | Low | Moderate |
Chemical Properties: Lead vs Tin
Lead exhibits higher atomic number 82 and greater density compared to Tin's atomic number 50, impacting their chemical reactivity and stability. Lead's oxidation states commonly range from +2 to +4, with +2 being more stable due to inert pair effect, while Tin exhibits +2 and +4 states with better stability in +4, enabling diverse compound formation. Both metals form oxides and salts, but Tin's oxides are more amphoteric, reflecting its position closer to nonmetals in the periodic table compared to Lead's more metallic behavior.
Physical Characteristics Comparison
Lead exhibits a high density of 11.34 g/cm3 and a low melting point of 327.5degC, making it heavy yet easily malleable at moderate temperatures. Tin, with a lower density of 7.31 g/cm3 and a melting point of 231.9degC, presents greater ductility and a softer metal profile. Both metals have distinct physical properties that influence their applications, with lead being more robust and tin favored for its flexibility and corrosion resistance.
Historical Uses of Lead and Tin
Lead has been historically favored for plumbing, paints, and ammunition due to its malleability and low melting point, while tin's primary historical use was in the production of bronze alloys and food preservation through tin plating. Ancient civilizations extensively utilized lead in water pipes and cosmetics despite its toxicity, whereas tin's corrosion resistance made it valuable for coating iron to prevent rust. The distinct properties of lead and tin shaped their roles in industrial and artisanal applications throughout history.
Manufacturing Processes and Methods
Lead and tin differ significantly in manufacturing processes due to their distinct melting points and material properties; lead melts at 327.5degC, facilitating low-temperature casting and molding, while tin melts at 231.9degC, allowing finer detail in soldering applications. Lead manufacturing often involves bulk casting and shaping for batteries and radiation shielding, whereas tin is primarily used in plating, soldering, and coating to prevent corrosion and enhance electrical connectivity. The choice between lead and tin in manufacturing hinges on factors like toxicity, mechanical strength, and environmental regulations influencing process methods.
Durability and Longevity
Lead exhibits superior durability in harsh environments compared to tin due to its higher corrosion resistance and density, which enhances its longevity in structural applications. Tin, while offering good corrosion resistance in specific conditions, tends to wear out faster under mechanical stress and prolonged exposure to moisture. The longevity of lead makes it preferable for long-term uses such as roofing, radiation shielding, and batteries where durability is critical.
Environmental Impact and Toxicity
Lead exhibits significantly higher toxicity compared to tin, posing severe environmental and health risks through soil and water contamination. Tin, especially in its metallic form, is relatively less toxic and degrades more safely, making it a preferable choice for reducing hazardous waste. Environmental regulations increasingly restrict lead use due to its persistence and bioaccumulation potential, while tin-based alternatives offer safer options for electronics and soldering applications.
Cost and Market Availability
Lead tends to be more cost-effective than tin due to its lower extraction and processing expenses, making it a preferred material in budget-sensitive applications. Market availability of lead is generally stable with abundant global reserves, whereas tin faces supply constraints caused by limited primary sources and geopolitical factors. These economic and availability differences significantly impact material selection in industries such as electronics and construction.
Applications in Industry
Lead is widely used in industries such as battery manufacturing, radiation shielding, and cable sheathing due to its high density and corrosion resistance, while tin finds extensive application in soldering, plating, and food packaging for its non-toxic and anti-corrosive properties. Lead's superior weight and malleability make it ideal for automotive batteries and construction materials, whereas tin's ability to form alloys like bronze and its compatibility with electronics dominate in circuit board production. Both metals play crucial roles in industrial processes, but lead is preferred for heavy-duty protective uses, and tin is favored for environmentally safer, lightweight, and conductive applications.
Safety Regulations and Handling
Lead and tin exhibit distinct differences in safety regulations due to lead's toxic properties and potential health risks such as neurological damage and kidney issues. Regulatory agencies like OSHA and EPA impose strict handling guidelines on lead, including the use of protective equipment and controlled exposure limits, whereas tin generally has fewer restrictions and is considered safer for everyday use. Proper ventilation, hygiene, and disposal protocols are essential when working with lead to minimize environmental contamination and human exposure.
Choosing Between Lead and Tin
Choosing between lead and tin depends primarily on their applications and toxicity levels. Lead offers superior durability and density, making it ideal for batteries and radiation shielding, while tin provides excellent corrosion resistance and is favored in soldering and food packaging due to its non-toxic properties. Considering environmental impact, tin is safer for consumer products, whereas lead requires careful handling and disposal to prevent health hazards.
Lead vs Tin Infographic
 materialdif.com
materialdif.com