Lead oxide and lead dioxide are two distinct compounds with different chemical properties and applications. Lead oxide, commonly used in lead-acid batteries, acts as a key component in the paste that forms the electrodes. In contrast, lead dioxide serves as the active material on the positive plate of these batteries, providing higher oxidation states crucial for battery efficiency and performance.
Table of Comparison
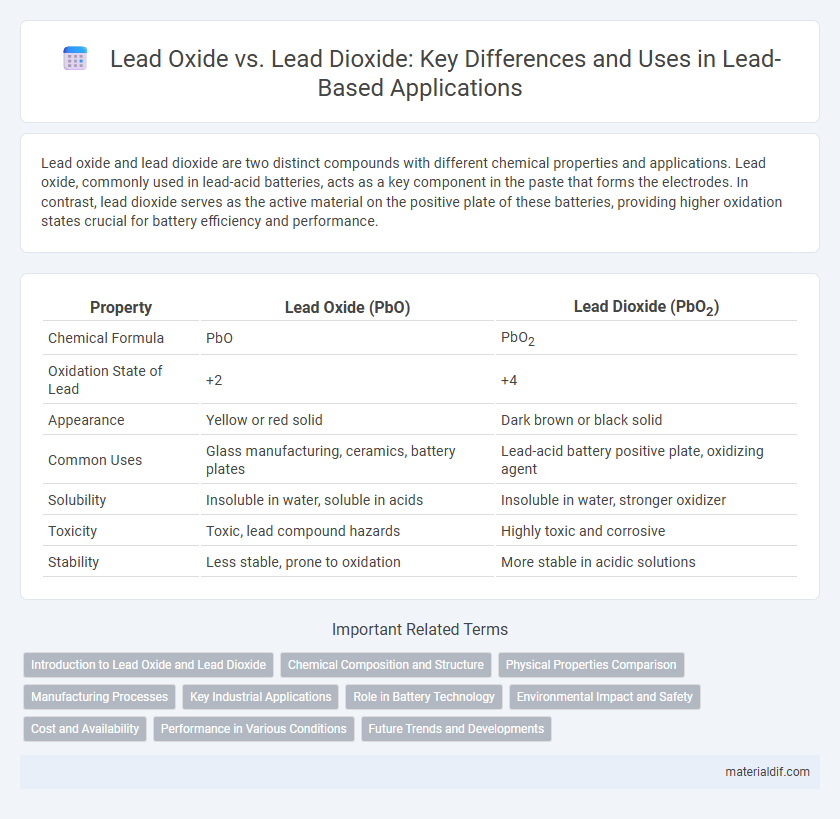
| Property | Lead Oxide (PbO) | Lead Dioxide (PbO2) |
|---|---|---|
| Chemical Formula | PbO | PbO2 |
| Oxidation State of Lead | +2 | +4 |
| Appearance | Yellow or red solid | Dark brown or black solid |
| Common Uses | Glass manufacturing, ceramics, battery plates | Lead-acid battery positive plate, oxidizing agent |
| Solubility | Insoluble in water, soluble in acids | Insoluble in water, stronger oxidizer |
| Toxicity | Toxic, lead compound hazards | Highly toxic and corrosive |
| Stability | Less stable, prone to oxidation | More stable in acidic solutions |
Introduction to Lead Oxide and Lead Dioxide
Lead oxide (PbO) and lead dioxide (PbO2) are two primary forms of lead compounds used in industrial applications, with distinct chemical properties and oxidation states. Lead oxide occurs mainly in two forms, litharge (yellow PbO) and massicot (red PbO), and serves as a precursor in the production of lead-acid batteries and pigments. Lead dioxide, a darker, more chemically stable compound, acts as a strong oxidizing agent in battery cathodes and various chemical syntheses, highlighting the importance of their complementary roles in lead chemistry.
Chemical Composition and Structure
Lead oxide (PbO) consists of lead in a +2 oxidation state combined with oxygen, forming a simple binary compound with a crystalline structure typically found in two polymorphs: litharge (tetragonal) and massicot (orthorhombic). Lead dioxide (PbO2) contains lead in a +4 oxidation state, exhibiting a more complex rutile-type tetragonal crystal lattice that imparts higher oxidative properties. The difference in oxidation states and crystal structures between PbO and PbO2 directly influences their chemical reactivity and applications in batteries and pigments.
Physical Properties Comparison
Lead oxide (PbO) exhibits a yellow or red crystalline form with a melting point of 888degC, while lead dioxide (PbO2) appears as a dark brown to nearly black solid with a decomposition temperature around 290degC. PbO has a tetragonal crystal structure and lower density (9.53 g/cm3) compared to PbO2, which has an orthorhombic structure and higher density (9.38 g/cm3). The distinct differences in color, crystal structure, melting point, and density between lead oxide and lead dioxide reflect their unique physical properties and stability under heat.
Manufacturing Processes
Lead oxide and lead dioxide differ significantly in their manufacturing processes due to their distinct chemical properties. Lead oxide is typically produced by controlled oxidation of molten lead or by the oxidation of lead metal at elevated temperatures, often through processes such as the Barton process or the ball mill process. In contrast, lead dioxide is commonly synthesized via electrochemical oxidation of lead anodes in sulfuric acid solutions, resulting in a highly crystalline and stable compound used primarily in lead-acid batteries.
Key Industrial Applications
Lead oxide is widely used in the manufacturing of lead-acid batteries due to its excellent electrochemical properties, playing a critical role in energy storage systems. Lead dioxide serves as a powerful oxidizing agent in the production of lead-acid battery cathodes and is essential in the electroplating and glass manufacturing industries. Both compounds are integral to industrial processes involving corrosion resistance, pigment production, and chemical synthesis.
Role in Battery Technology
Lead oxide (PbO) serves as a fundamental active material in the manufacture of lead-acid battery plates, facilitating the electrochemical reaction during discharge and charge cycles. Lead dioxide (PbO2), found on the positive plate in lead-acid batteries, acts as a strong oxidizing agent, enhancing energy density and overall battery efficiency. Both compounds play critical roles in the battery's ability to store and release electrical energy through reversible redox reactions.
Environmental Impact and Safety
Lead oxide poses significant environmental risks due to its higher solubility and potential for soil and water contamination, making it more bioavailable and toxic to aquatic life. Lead dioxide, while chemically more stable and less soluble, can still release harmful lead ions under acidic conditions, posing risks during manufacturing and disposal. Both compounds require stringent handling and disposal protocols to minimize environmental contamination and human exposure.
Cost and Availability
Lead oxide is generally more affordable and widely available due to its extensive industrial use and simpler production methods, making it the preferred choice for large-scale manufacturing. In contrast, lead dioxide is more expensive and less readily available, mainly produced through specialized chemical processes, limiting its use to specific applications like lead-acid battery cathodes. Market demand and raw material sources significantly influence the cost disparity between lead oxide and lead dioxide.
Performance in Various Conditions
Lead oxide and lead dioxide exhibit distinct performance characteristics under various conditions critical to battery efficiency and durability. Lead dioxide (PbO2) demonstrates superior electrochemical stability and higher energy density, making it ideal for the positive plate in lead-acid batteries, especially in high-load or deep-cycle applications. In contrast, lead oxide (PbO) primarily functions as a precursor in paste preparation, offering better charge acceptance at moderate temperatures but lower overall conductivity and corrosion resistance compared to lead dioxide.
Future Trends and Developments
Lead dioxide (PbO2) is gaining prominence in energy storage technologies due to its higher oxidation state, enhancing battery performance and longevity compared to lead oxide (PbO). Future trends emphasize the development of nanostructured lead dioxide electrodes to improve electrochemical efficiency and reduce environmental impact. Advances in sustainable recycling methods for both lead oxide and lead dioxide aim to support circular economy initiatives in the lead-acid battery industry.
Lead oxide vs Lead dioxide Infographic
 materialdif.com
materialdif.com