Lead oxide and zinc oxide are both metal oxides used in various industrial applications, but they differ significantly in toxicity and environmental impact. Lead oxide is highly toxic and poses serious health risks, making zinc oxide a safer alternative in products such as paints, rubber, and cosmetics. Zinc oxide also offers excellent UV protection and antibacterial properties, enhancing its versatility compared to lead oxide.
Table of Comparison
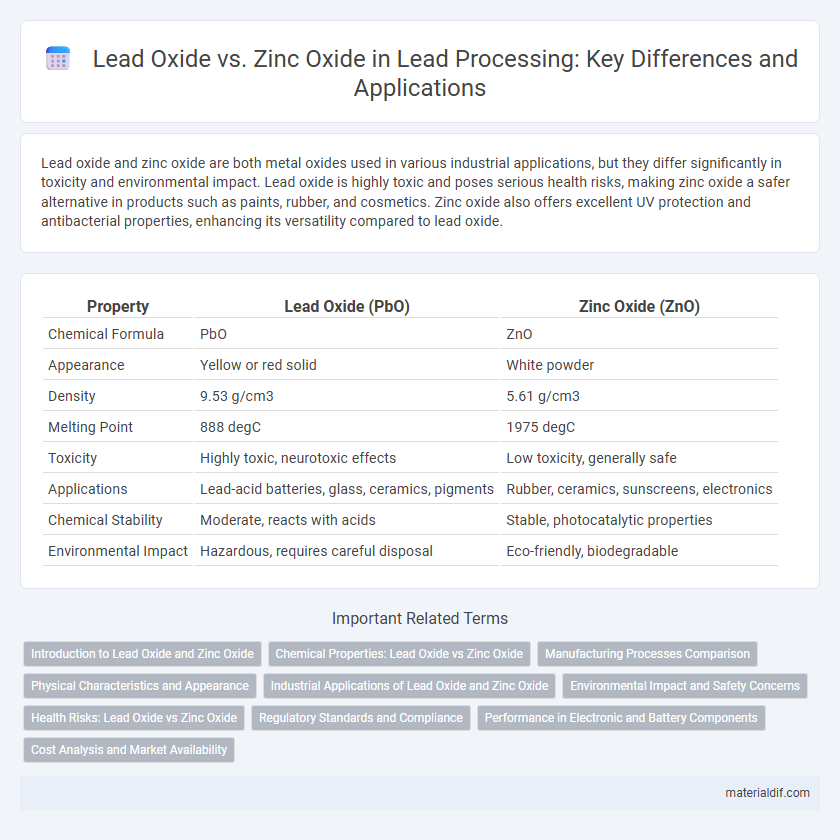
| Property | Lead Oxide (PbO) | Zinc Oxide (ZnO) |
|---|---|---|
| Chemical Formula | PbO | ZnO |
| Appearance | Yellow or red solid | White powder |
| Density | 9.53 g/cm3 | 5.61 g/cm3 |
| Melting Point | 888 degC | 1975 degC |
| Toxicity | Highly toxic, neurotoxic effects | Low toxicity, generally safe |
| Applications | Lead-acid batteries, glass, ceramics, pigments | Rubber, ceramics, sunscreens, electronics |
| Chemical Stability | Moderate, reacts with acids | Stable, photocatalytic properties |
| Environmental Impact | Hazardous, requires careful disposal | Eco-friendly, biodegradable |
Introduction to Lead Oxide and Zinc Oxide
Lead oxide, a heavy metal compound with the chemical formula PbO, is commonly used in batteries, glass manufacturing, and pigments due to its semiconductor properties and high density. Zinc oxide (ZnO) is a versatile oxide known for its applications in sunscreens, rubber manufacturing, and electronics, valued for its UV protection, antibacterial properties, and wide bandgap semiconductor behavior. Both oxides play crucial roles in industrial processes, with lead oxide exhibiting toxicological risks while zinc oxide is generally considered safer and environmentally friendly.
Chemical Properties: Lead Oxide vs Zinc Oxide
Lead oxide (PbO) exhibits amphoteric behavior, reacting with both acids and bases, and has a higher melting point around 888degC compared to zinc oxide's (ZnO) melting point of approximately 1975degC. Zinc oxide is a wide-bandgap semiconductor with significant photocatalytic properties and exhibits strong ionic character, while lead oxide shows mixed ionic-covalent bonding and is less stable under high temperatures. Both oxides form basic oxides but lead oxide can exist in multiple forms (litharge and massicot) affecting its reactivity, whereas zinc oxide predominantly crystallizes in the wurtzite structure providing consistent chemical stability.
Manufacturing Processes Comparison
Lead oxide production involves the oxidation of metallic lead through the Barton Pot or Ball Mill process, enabling precise control over particle size and chemical composition for battery applications. Zinc oxide manufacturing typically employs either the indirect (French) process, where zinc vapor is oxidized in air at high temperatures, or the direct (American) process, which involves the vaporization of zinc metal in a furnace followed by oxidation, optimizing purity and particle morphology for consistent performance. Differences in thermal treatment, raw material handling, and scale-up flexibility significantly affect product quality and industrial applicability in electronics and ceramics manufacturing.
Physical Characteristics and Appearance
Lead oxide typically appears as a dense, yellow to reddish-brown powder with a high melting point and significant weight, exhibiting poor solubility in water. Zinc oxide manifests as a white, powdery substance with a lower density and melting point compared to lead oxide, known for its non-toxicity and excellent UV absorption properties. Both oxides have distinct crystalline structures influencing their applications in pigments, ceramics, and rubber industries.
Industrial Applications of Lead Oxide and Zinc Oxide
Lead oxide is widely used in the manufacturing of lead-acid batteries, glass, and ceramics due to its excellent electrical conductivity and chemical stability. Zinc oxide plays a critical role in rubber production, cosmetics, and pharmaceuticals, where its antimicrobial and UV-protection properties are essential. Industrial applications of lead oxide emphasize energy storage and glass production, whereas zinc oxide's applications center on protective coatings and healthcare products.
Environmental Impact and Safety Concerns
Lead oxide poses significant environmental hazards due to its toxicity and potential to cause soil and water contamination, leading to severe health risks such as neurological damage. Zinc oxide, in contrast, is considered much safer and environmentally benign, widely used in products like sunscreens and paints without the same level of regulatory restrictions. Proper handling and disposal of lead oxide are critical to mitigate its hazardous effects, whereas zinc oxide offers a more sustainable option with minimal environmental impact.
Health Risks: Lead Oxide vs Zinc Oxide
Lead oxide poses significant health risks due to its high toxicity and potential to cause neurological damage, respiratory issues, and kidney problems upon exposure. Zinc oxide, in contrast, is generally considered safe and is widely used in sunscreens, cosmetics, and medical applications with minimal toxicity and low risk of adverse health effects. Prolonged or excessive exposure to lead oxide requires stringent safety measures, whereas zinc oxide's health risks are comparatively negligible under normal usage.
Regulatory Standards and Compliance
Lead oxide, classified as a toxic heavy metal compound, faces stringent regulatory standards including limits set by the Environmental Protection Agency (EPA) and the European Union's REACH regulation, restricting its use in consumer products due to health and environmental risks. Zinc oxide is widely recognized as safe and compliant with regulations set by the FDA for use in cosmetics and sunscreens, as well as OSHA standards for occupational exposure. Compliance with these regulatory frameworks ensures that zinc oxide remains a preferred additive in various industries, while lead oxide is subject to strict handling and disposal protocols to mitigate toxicological hazards.
Performance in Electronic and Battery Components
Lead oxide exhibits high electrical conductivity and excellent charge storage capacity, making it ideal for lead-acid battery electrodes with superior energy density and cycle stability. Zinc oxide offers wide bandgap and high electron mobility, enhancing performance in varistors, transparent conductive films, and battery anodes with fast charge-discharge rates. Comparing both oxides, lead oxide is preferred for high-capacity energy storage, while zinc oxide excels in electronic applications requiring semiconducting properties and rapid electron transport.
Cost Analysis and Market Availability
Lead oxide generally costs less than zinc oxide due to cheaper raw materials and simpler manufacturing processes, making it more attractive for budget-sensitive applications. Zinc oxide, while more expensive, offers superior properties like higher stability and non-toxicity, justifying its price in premium markets. Market availability favors zinc oxide, with wider distribution globally and increasing demand in electronics and cosmetics, whereas lead oxide faces regulatory restrictions limiting its accessibility.
Lead oxide vs Zinc oxide Infographic
 materialdif.com
materialdif.com