Lead oxide is primarily used in the production of batteries, ceramics, and glass due to its stable chemical properties, whereas lead nitrate is a soluble compound frequently employed in laboratories and industrial processes such as dyeing and pyrotechnics. Lead oxide tends to form a yellow or red powder, while lead nitrate appears as colorless crystals highly soluble in water. Understanding the distinct chemical behavior and applications of lead oxide versus lead nitrate is crucial for safe handling and effective utilization in various industries.
Table of Comparison
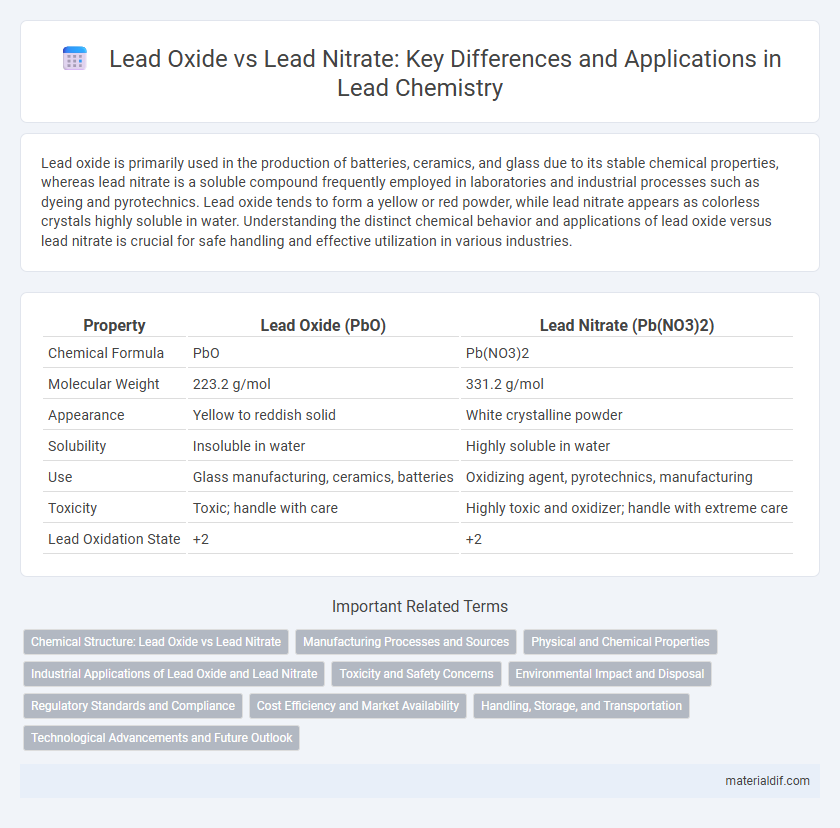
| Property | Lead Oxide (PbO) | Lead Nitrate (Pb(NO3)2) |
|---|---|---|
| Chemical Formula | PbO | Pb(NO3)2 |
| Molecular Weight | 223.2 g/mol | 331.2 g/mol |
| Appearance | Yellow to reddish solid | White crystalline powder |
| Solubility | Insoluble in water | Highly soluble in water |
| Use | Glass manufacturing, ceramics, batteries | Oxidizing agent, pyrotechnics, manufacturing |
| Toxicity | Toxic; handle with care | Highly toxic and oxidizer; handle with extreme care |
| Lead Oxidation State | +2 | +2 |
Chemical Structure: Lead Oxide vs Lead Nitrate
Lead oxide primarily consists of PbO, featuring a crystalline lattice where lead ions are bonded to oxygen ions in a simple ionic structure, whereas lead nitrate, Pb(NO3)2, contains lead ions coordinated with nitrate anions, forming a more complex ionic compound with covalently bonded nitrate groups. The oxidation state of lead in lead oxide is generally +2, while in lead nitrate it is also +2, but the presence of nitrate groups introduces additional oxygen atoms and molecular complexity. These structural differences influence their solubility, reactivity, and applications in various industrial and chemical processes.
Manufacturing Processes and Sources
Lead oxide is primarily manufactured through the oxidation of lead metal at elevated temperatures, often using controlled air flow in rotary kilns or furnaces, whereas lead nitrate is synthesized by reacting lead carbonate or lead metal with nitric acid in a wet chemical process. Lead oxide production involves thermal decomposition and oxidation steps targeting PbO or Pb3O4 compounds, while lead nitrate formation emphasizes aqueous solution chemistry and precise temperature control to yield Pb(NO3)2 crystals. Industrial sources for lead oxide dominate in smelting and battery recycling plants, while lead nitrate is chiefly produced in chemical manufacturing facilities specializing in inorganic salts.
Physical and Chemical Properties
Lead oxide appears as a yellow or red solid with a high melting point around 888degC, exhibiting amphoteric behavior by reacting with both acids and bases, while lead nitrate is a white crystalline salt soluble in water and decomposes upon heating to release nitrogen dioxide and lead oxide. Chemically, lead oxide exists mainly in two forms, PbO and Pb3O4, showing different oxidation states of lead (+2 and +4), whereas lead nitrate contains lead in the +2 oxidation state combined with nitrate ions, making it a strong oxidizer. The contrasting solubility and thermal properties influence their applications, with lead nitrate commonly used in pyrotechnics and oxidizing agents and lead oxide in battery electrodes and glass manufacturing.
Industrial Applications of Lead Oxide and Lead Nitrate
Lead oxide, primarily used in the manufacture of lead-acid batteries and glass production, serves as a vital component in industrial applications due to its excellent electrical conductivity and chemical stability. Lead nitrate, characterized by its role as an oxidizing agent, finds extensive use in the synthesis of other lead compounds, pyrotechnics, and as a reagent in laboratory processes. Both compounds are critical in various sectors, with lead oxide dominating energy storage solutions and lead nitrate facilitating chemical manufacturing and explosives.
Toxicity and Safety Concerns
Lead oxide and lead nitrate are both highly toxic compounds, with lead nitrate posing a greater acute toxicity risk due to its higher solubility and bioavailability. Lead oxide primarily causes chronic health effects through inhalation or ingestion, leading to neurotoxicity, kidney damage, and anemia, while lead nitrate's water solubility increases the risk of environmental contamination and ingestion exposure. Strict safety measures, including proper handling, storage, and personal protective equipment, are essential to minimize exposure and prevent lead poisoning from both substances.
Environmental Impact and Disposal
Lead oxide and lead nitrate both pose significant environmental risks due to their toxicity and heavy metal content. Lead oxide is less soluble but tends to accumulate in soils and sediments, causing long-term contamination and bioaccumulation in ecosystems. Lead nitrate, being highly soluble, can rapidly contaminate water sources and requires specialized disposal methods to prevent heavy metal leaching and groundwater pollution.
Regulatory Standards and Compliance
Lead oxide and lead nitrate are subject to stringent regulatory standards due to their toxicity and environmental impact, with agencies like the EPA and OSHA enforcing exposure limits and handling protocols. Lead oxide is often regulated as a hazardous waste under RCRA guidelines, requiring proper disposal to prevent soil and water contamination. Lead nitrate's solubility enhances its bioavailability, making compliance with storage, transportation, and usage regulations crucial to minimize risks in industrial and laboratory settings.
Cost Efficiency and Market Availability
Lead oxide offers greater cost efficiency due to its lower production costs and widespread industrial use, making it more affordable in bulk compared to lead nitrate. Lead nitrate, while more specialized and costly, is less abundant in the market with limited suppliers, impacting its availability for large-scale applications. Market demand for lead oxide in battery manufacturing and glass production supports its stable pricing and accessibility, contrasting with lead nitrate's niche usage in pyrotechnics and laboratory settings.
Handling, Storage, and Transportation
Lead oxide requires careful handling due to its toxicity and dust form, necessitating sealed containers and proper ventilation to minimize inhalation risks. Lead nitrate demands even stricter storage conditions as it is highly soluble and an oxidizing agent, requiring cool, dry, and well-ventilated areas away from combustible materials. Transportation of both compounds mandates compliance with hazardous materials regulations, using secure, labeled containers to prevent leaks and environmental contamination.
Technological Advancements and Future Outlook
Lead oxide demonstrates significant potential in battery technology due to its superior electrical conductivity and stability, making it a key material in the development of advanced lead-acid batteries. Lead nitrate, while primarily used in chemical synthesis and pigment production, is being reexamined for its role in nanomaterial fabrication and environmental remediation technologies. Future advancements are likely to enhance the performance and sustainability of lead-based compounds, leveraging their unique chemical properties for innovative energy storage and environmental applications.
Lead oxide vs Lead nitrate Infographic
 materialdif.com
materialdif.com