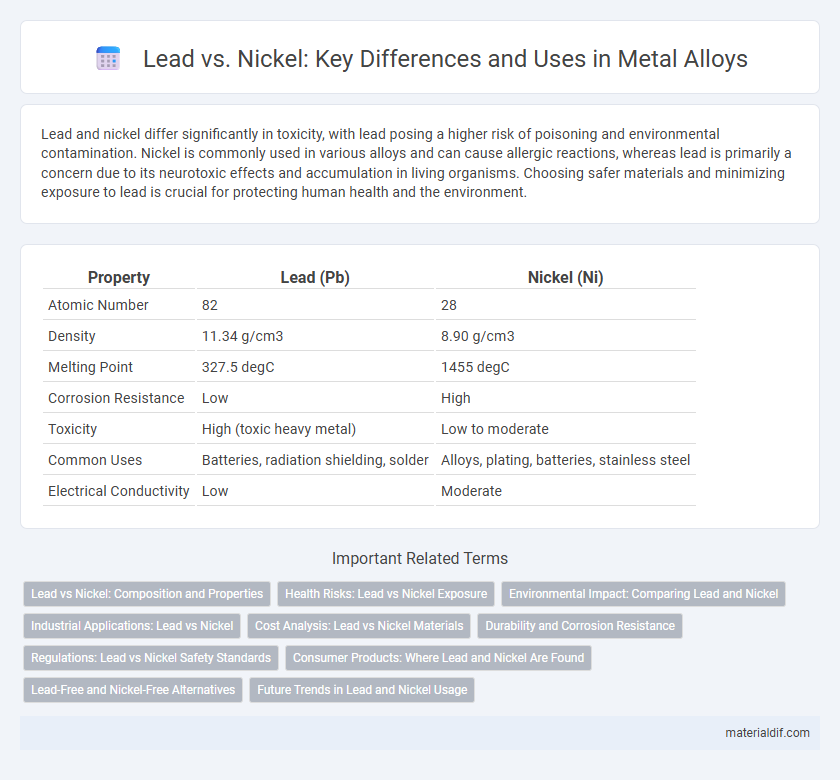
Lead and nickel differ significantly in toxicity, with lead posing a higher risk of poisoning and environmental contamination. Nickel is commonly used in various alloys and can cause allergic reactions, whereas lead is primarily a concern due to its neurotoxic effects and accumulation in living organisms. Choosing safer materials and minimizing exposure to lead is crucial for protecting human health and the environment.
Table of Comparison
| Property | Lead (Pb) | Nickel (Ni) |
|---|---|---|
| Atomic Number | 82 | 28 |
| Density | 11.34 g/cm3 | 8.90 g/cm3 |
| Melting Point | 327.5 degC | 1455 degC |
| Corrosion Resistance | Low | High |
| Toxicity | High (toxic heavy metal) | Low to moderate |
| Common Uses | Batteries, radiation shielding, solder | Alloys, plating, batteries, stainless steel |
| Electrical Conductivity | Low | Moderate |
Lead vs Nickel: Composition and Properties
Lead primarily consists of a dense, soft metal with high malleability and excellent corrosion resistance, whereas nickel is a hard, silvery-white metal known for its strength and resistance to oxidation. Lead's atomic number is 82, with a density of 11.34 g/cm3 and melting point of 327.5degC, contrasting with nickel's atomic number 28, density of 8.90 g/cm3, and higher melting point of 1455degC. The differing physical and chemical properties make lead more suitable for applications like radiation shielding and batteries, while nickel is favored in stainless steel production and high-temperature alloying.
Health Risks: Lead vs Nickel Exposure
Lead exposure poses severe health risks, including neurological damage, kidney dysfunction, and developmental delays in children due to its high toxicity and bioaccumulation. Nickel exposure primarily causes skin allergies, respiratory issues, and has been classified as a potential carcinogen with prolonged inhalation. Both metals require strict regulatory limits to minimize chronic health effects and occupational hazards.
Environmental Impact: Comparing Lead and Nickel
Lead toxicity poses significant environmental hazards due to its persistence in soil and water, leading to bioaccumulation and severe health risks for wildlife and humans. Nickel, while less toxic, contributes to soil and water contamination through industrial discharge, causing allergic reactions and ecological disruptions in aquatic systems. Effective waste management and recycling practices are critical in mitigating the environmental impact of both metals.
Industrial Applications: Lead vs Nickel
Lead is extensively used in lead-acid batteries, radiation shielding, and cable sheathing due to its high density and corrosion resistance, making it essential in automotive and electrical industries. Nickel excels in stainless steel production, electroplating, and battery cathodes, offering superior strength, corrosion resistance, and heat tolerance critical for aerospace, chemical processing, and electronics. Industrial applications favor lead for heavy-duty protection and cost-effectiveness, while nickel is preferred for durability and performance in high-stress environments.
Cost Analysis: Lead vs Nickel Materials
Lead offers a significantly lower material cost compared to nickel, making it a more economical choice for applications where budget constraints are critical. Nickel's higher price is driven by its superior corrosion resistance and durability, which justify the investment depending on performance requirements. Cost analysis must weigh initial material expenses against long-term benefits such as maintenance and lifespan to determine the optimal choice between lead and nickel.
Durability and Corrosion Resistance
Lead exhibits superior corrosion resistance compared to nickel, particularly in acidic and saline environments, making it ideal for applications requiring long-term durability. Nickel offers high mechanical strength but is more susceptible to oxidation and surface degradation over time. The choice between lead and nickel depends on environmental exposure, where lead ensures enhanced longevity in corrosive settings.
Regulations: Lead vs Nickel Safety Standards
Lead regulations are stricter due to its high toxicity and bioaccumulative properties, prompting limits in consumer products like paint and plumbing. Nickel safety standards focus on reducing allergic reactions, with specific guidelines in jewelry and skin contact products under REACH regulations in Europe. Both metals require compliance with workplace exposure limits set by agencies like OSHA to ensure human health protection.
Consumer Products: Where Lead and Nickel Are Found
Lead is commonly found in batteries, ammunition, and certain types of paints used in consumer products, while nickel is prevalent in stainless steel appliances, coins, and rechargeable batteries. Consumer electronics often contain nickel due to its corrosion resistance and strength, whereas lead persists in older products and some plumbing materials. The difference in toxicity between lead and nickel influences their regulatory use and consumer safety standards.
Lead-Free and Nickel-Free Alternatives
Lead-free and nickel-free alternatives are essential for reducing health risks associated with traditional lead and nickel components in electronics and jewelry. Tin, silver, and copper alloys serve as effective lead-free options, offering comparable conductivity and durability without the toxicity. For nickel-free solutions, materials such as titanium, stainless steel, and hypoallergenic plastics minimize allergic reactions while maintaining structural integrity and aesthetic appeal.
Future Trends in Lead and Nickel Usage
Future trends indicate a gradual decline in lead usage due to stringent environmental regulations and the rise of safer alternatives in battery technology. Conversely, nickel demand is expected to surge, driven by its critical role in electric vehicle batteries and renewable energy storage systems. Innovations in nickel refining and recycling processes aim to meet this increasing demand while reducing environmental impact.
Lead vs Nickel Infographic
 materialdif.com
materialdif.com