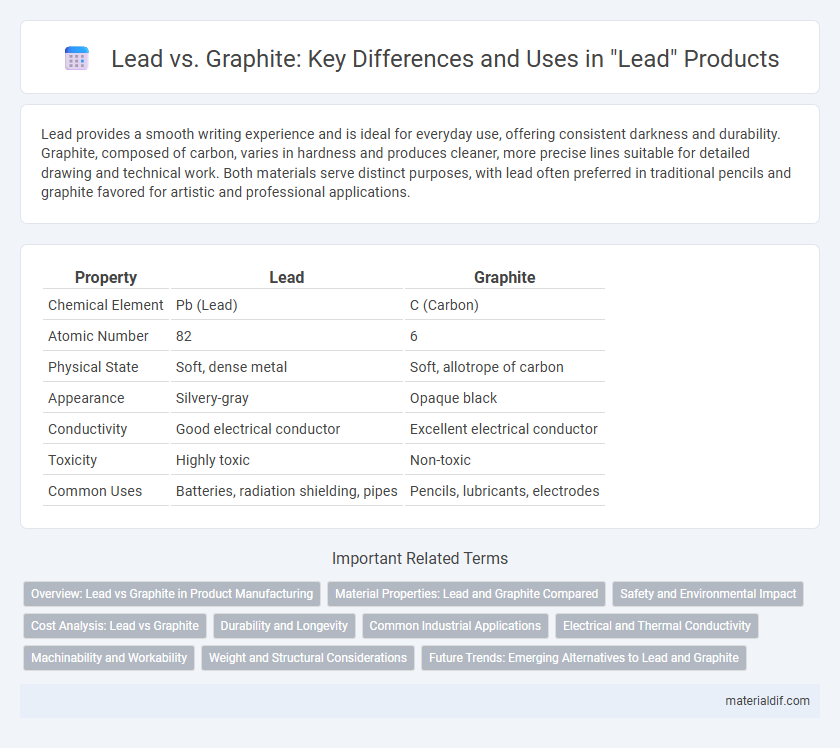
Lead provides a smooth writing experience and is ideal for everyday use, offering consistent darkness and durability. Graphite, composed of carbon, varies in hardness and produces cleaner, more precise lines suitable for detailed drawing and technical work. Both materials serve distinct purposes, with lead often preferred in traditional pencils and graphite favored for artistic and professional applications.
Table of Comparison
| Property | Lead | Graphite |
|---|---|---|
| Chemical Element | Pb (Lead) | C (Carbon) |
| Atomic Number | 82 | 6 |
| Physical State | Soft, dense metal | Soft, allotrope of carbon |
| Appearance | Silvery-gray | Opaque black |
| Conductivity | Good electrical conductor | Excellent electrical conductor |
| Toxicity | Highly toxic | Non-toxic |
| Common Uses | Batteries, radiation shielding, pipes | Pencils, lubricants, electrodes |
Overview: Lead vs Graphite in Product Manufacturing
Lead and graphite differ significantly in product manufacturing due to their distinct chemical and physical properties; lead is a dense, malleable metal commonly used in batteries, radiation shielding, and weights, while graphite, a crystalline form of carbon, serves as a lubricant, an electrode material, and a key component in pencils and batteries. Graphite's high thermal conductivity and chemical inertness make it ideal for high-temperature applications and electrochemical uses, contrasting with lead's toxicity and softness, which limit its applications and necessitate careful handling. The choice between lead and graphite in manufacturing hinges on factors like conductivity, durability, environmental impact, and specific product requirements.
Material Properties: Lead and Graphite Compared
Lead is a dense, malleable metal with a low melting point of 327.5degC and excellent corrosion resistance, making it suitable for applications requiring durability and flexibility. Graphite is a form of carbon with a layered crystalline structure, notable for its high electrical conductivity, chemical stability, and lubricating properties. While lead is soft and heavy, graphite is lightweight and brittle, and its unique anisotropic properties enable uses in batteries, lubricants, and electrodes beyond traditional lead applications.
Safety and Environmental Impact
Lead poses significant health risks due to its toxicity, potentially causing neurological damage and environmental contamination through soil and water pollution. Graphite, by contrast, is chemically inert and non-toxic, making it a safer alternative in applications like pencils and batteries. Environmental regulations increasingly restrict lead usage to mitigate its adverse effects, emphasizing the shift toward eco-friendly materials such as graphite.
Cost Analysis: Lead vs Graphite
Lead is significantly more affordable than graphite, making it a cost-effective choice for applications such as batteries and radiation shielding. Graphite, while more expensive, offers superior electrical conductivity and thermal resistance, justifying its higher price in industrial uses like electrodes and lubricants. The cost differential influences material selection depending on budget constraints and performance requirements.
Durability and Longevity
Lead outlasts graphite in durability due to its higher resistance to breaking under pressure, making it ideal for applications requiring long-lasting writing or marking tools. Graphite, while smoother and lighter, tends to wear down faster and is more prone to smudging, reducing its longevity in comparison to lead-based materials. The durability advantage of lead ensures extended use and consistent performance in industrial and artistic environments.
Common Industrial Applications
Lead is widely used in batteries, radiation shielding, and ammunition due to its high density and corrosion resistance, while graphite excels in applications requiring high thermal conductivity and electrical conductivity, such as electrodes, lubricants, and refractory materials. Lead compounds are prevalent in pigments and stabilizers for plastics, whereas graphite's layered structure makes it ideal for use in nuclear reactors and advanced composites. Industrial sectors rely on lead for its protective qualities and graphite for mechanical and thermal performance, highlighting their distinct but complementary roles.
Electrical and Thermal Conductivity
Lead exhibits lower electrical conductivity, approximately 4.8 x 10^6 S/m, compared to graphite's anisotropic conductivity, which can reach up to 10^5 S/m along the planes and varies significantly across different directions. Thermal conductivity of lead is also relatively low, around 35 W/m*K, whereas graphite demonstrates high thermal conductivity in-plane, up to 2000 W/m*K, due to its layered crystal structure. These differences make graphite more efficient for applications requiring high electrical and thermal conductivity, while lead's properties suit uses demanding lower conductivity and higher density.
Machinability and Workability
Lead exhibits superior machinability compared to graphite due to its ductile and malleable nature, allowing easy cutting, shaping, and drilling without excessive wear on tools. Graphite, being brittle and abrasive, tends to cause more tool wear and requires specialized equipment for precise machining processes. The workability of lead is enhanced by its softness and ability to absorb shock, whereas graphite is primarily used in applications where lubrication and thermal resistance are critical despite its limited formability.
Weight and Structural Considerations
Lead is significantly denser than graphite, with a density of approximately 11.34 g/cm3 compared to graphite's 2.26 g/cm3, making lead much heavier for the same volume. Structurally, graphite is composed of layers of carbon atoms arranged in hexagonal lattices, providing lightweight yet strong material properties, while lead's metallic bonding results in a softer, more malleable, and less structurally rigid substance. These differences influence their applications, with graphite preferred for lightweight structural uses and lead utilized where high density and malleability are required.
Future Trends: Emerging Alternatives to Lead and Graphite
Future trends in material science are exploring emerging alternatives to lead and graphite, such as lithium-ion batteries utilizing silicon anodes and solid-state electrolytes for enhanced energy density and safety. Research into graphene and other carbon-based nanomaterials aims to outperform graphite in conductivity and durability within battery technology. Regulatory pressures and sustainability concerns drive innovation toward eco-friendly, high-performance substitutes like sodium-ion and magnesium-ion battery components.
Lead vs Graphite Infographic
 materialdif.com
materialdif.com