Lead anodes and lead cathodes serve distinct functions in electrochemical processes, with lead anodes acting as the site of oxidation and lead cathodes facilitating reduction. The choice between a lead anode and cathode influences cell efficiency, corrosion resistance, and overall battery performance. Understanding the electrochemical properties of lead anodes versus lead cathodes is essential for optimizing applications such as lead-acid batteries and electroplating.
Table of Comparison
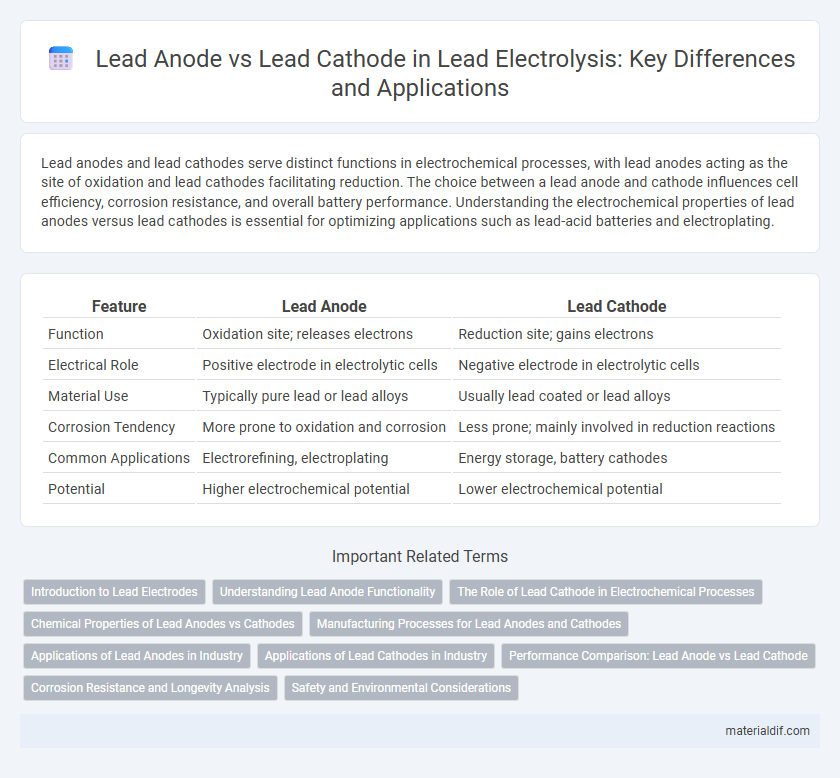
| Feature | Lead Anode | Lead Cathode |
|---|---|---|
| Function | Oxidation site; releases electrons | Reduction site; gains electrons |
| Electrical Role | Positive electrode in electrolytic cells | Negative electrode in electrolytic cells |
| Material Use | Typically pure lead or lead alloys | Usually lead coated or lead alloys |
| Corrosion Tendency | More prone to oxidation and corrosion | Less prone; mainly involved in reduction reactions |
| Common Applications | Electrorefining, electroplating | Energy storage, battery cathodes |
| Potential | Higher electrochemical potential | Lower electrochemical potential |
Introduction to Lead Electrodes
Lead anodes and lead cathodes serve distinct functions in electrochemical cells, with lead anodes acting as the site for oxidation and lead cathodes facilitating reduction reactions. In lead-acid batteries, lead anodes typically consist of spongy lead or lead dioxide, which influence battery efficiency and lifespan. The electrochemical properties of lead electrodes, including their corrosion resistance and conductivity, are critical factors in their performance and application stability.
Understanding Lead Anode Functionality
Lead anodes serve as the sacrificial electrode in electrochemical cells, undergoing oxidation to protect the cathode from corrosion. Their primary function involves releasing lead ions into the electrolyte, thereby maintaining the cell's electrical balance and ensuring consistent conductivity. This controlled oxidation process differentiates lead anodes from lead cathodes, which instead attract ions and are involved in reduction reactions within the cell.
The Role of Lead Cathode in Electrochemical Processes
The lead cathode plays a crucial role in electrochemical processes by serving as the site for reduction reactions where lead ions gain electrons to form metallic lead. Its high conductivity and corrosion resistance enhance the efficiency and longevity of electrochemical cells, particularly in lead-acid batteries and electrowinning applications. Optimizing lead cathode surfaces improves charge transfer rates and overall cell performance, directly impacting energy storage and metal recovery systems.
Chemical Properties of Lead Anodes vs Cathodes
Lead anodes exhibit high oxidation potential, facilitating the release of lead ions during electrochemical reactions, which enhances their effectiveness in corrosion protection and electroplating processes. In contrast, lead cathodes demonstrate strong reduction properties, promoting the deposition of lead ions from solution and ensuring durability in battery applications, such as lead-acid cells. The distinct chemical behaviors of lead anodes and cathodes arise from their roles in redox reactions, with anodes undergoing oxidation and cathodes undergoing reduction, influencing their stability and functionality in various electrochemical systems.
Manufacturing Processes for Lead Anodes and Cathodes
Manufacturing processes for lead anodes involve casting pure lead or lead alloys into specific shapes, followed by surface treatment to enhance corrosion resistance and conductivity. Lead cathode production typically requires electrodeposition, where lead ions are reduced and deposited onto a substrate, forming a uniform, dense layer crucial for battery performance. Both processes emphasize precise control of material purity, surface morphology, and dimensional accuracy to ensure optimal electrochemical efficiency in applications like lead-acid batteries.
Applications of Lead Anodes in Industry
Lead anodes are widely used in electroplating, corrosion protection, and electrochemical synthesis due to their high conductivity and resistance to corrosion in acidic and saline environments. In industries such as metal finishing, water treatment, and battery manufacturing, lead anodes facilitate efficient current transfer and maintain durability under harsh operating conditions. Their predominant applications include chlor-alkali production, electrorefining of metals, and cathodic protection systems for pipelines and marine structures.
Applications of Lead Cathodes in Industry
Lead cathodes are widely used in the electroplating industry to provide a stable, corrosion-resistant surface for depositing metals like copper, nickel, and silver. In battery manufacturing, lead cathodes serve as critical components in lead-acid batteries, enhancing durability and conductivity. Their excellent resistance to chemical erosion also makes them ideal for use in industrial electrolytic processes such as metal refining and wastewater treatment.
Performance Comparison: Lead Anode vs Lead Cathode
Lead anodes exhibit higher corrosion rates, enhancing oxidation efficiency, while lead cathodes provide superior reduction stability and lower hydrogen overpotential, improving overall cell durability. The performance of lead anodes is characterized by their rapid electron emission, making them ideal for initiating electrochemical reactions, whereas lead cathodes excel in maintaining consistent current density with minimal degradation. Comparative studies show lead cathodes deliver prolonged service life and energy efficiency in electrolysis applications compared to lead anodes.
Corrosion Resistance and Longevity Analysis
Lead anodes exhibit superior corrosion resistance due to their ability to form a stable oxide layer, which significantly enhances longevity in electrochemical applications. In contrast, lead cathodes experience less oxidative degradation but can suffer from mechanical wear and hydrogen embrittlement over time. The longevity of lead anodes in environments like marine and industrial electroplating settings consistently outperforms lead cathodes, reducing maintenance frequency and operational costs.
Safety and Environmental Considerations
Lead anodes and lead cathodes differ in their safety and environmental impact, with lead anodes typically posing higher risks due to their potential for lead dust and metal exposure during manufacturing and use. Proper handling and disposal protocols are crucial to minimize lead contamination in soil and water, especially since lead is a toxic heavy metal with significant health hazards such as neurological damage. Employing sealed systems and advanced filtration technologies reduces lead emissions, enhancing workplace safety and environmental protection in industries utilizing lead electrodes.
Lead anode vs Lead cathode Infographic
 materialdif.com
materialdif.com