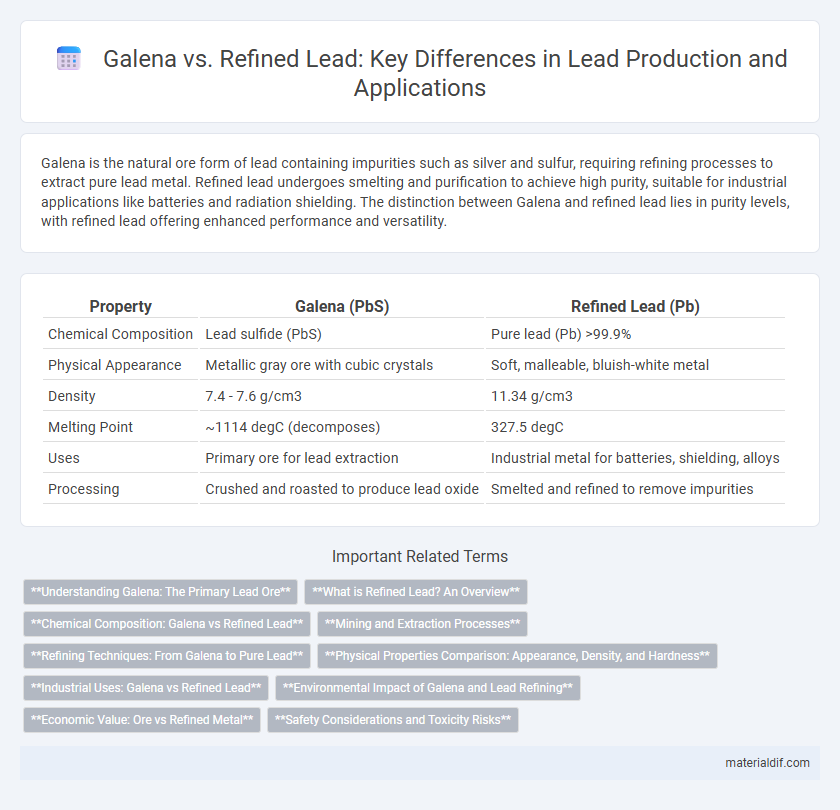
Galena is the natural ore form of lead containing impurities such as silver and sulfur, requiring refining processes to extract pure lead metal. Refined lead undergoes smelting and purification to achieve high purity, suitable for industrial applications like batteries and radiation shielding. The distinction between Galena and refined lead lies in purity levels, with refined lead offering enhanced performance and versatility.
Table of Comparison
| Property | Galena (PbS) | Refined Lead (Pb) |
|---|---|---|
| Chemical Composition | Lead sulfide (PbS) | Pure lead (Pb) >99.9% |
| Physical Appearance | Metallic gray ore with cubic crystals | Soft, malleable, bluish-white metal |
| Density | 7.4 - 7.6 g/cm3 | 11.34 g/cm3 |
| Melting Point | ~1114 degC (decomposes) | 327.5 degC |
| Uses | Primary ore for lead extraction | Industrial metal for batteries, shielding, alloys |
| Processing | Crushed and roasted to produce lead oxide | Smelted and refined to remove impurities |
Understanding Galena: The Primary Lead Ore
Galena is the principal ore of lead, primarily composed of lead sulfide (PbS), making it the most significant source for lead extraction worldwide. Its high lead content, often exceeding 86%, allows for efficient refining processes to produce pure lead suitable for industrial applications. Understanding the mineralogical properties of galena is crucial for optimizing ore processing techniques and enhancing lead recovery rates.
What is Refined Lead? An Overview
Refined lead is a purified form of lead obtained through processes such as smelting and electrolysis, removing impurities found in raw galena ore. Galena, primarily lead sulfide (PbS), serves as the most important lead ore but contains contaminants that affect its quality. Refined lead boasts higher purity levels, making it ideal for applications in batteries, radiation shielding, and construction materials.
Chemical Composition: Galena vs Refined Lead
Galena primarily consists of lead sulfide (PbS), making it the most important ore for extracting lead, while refined lead is produced by smelting galena and other lead-bearing materials to remove impurities. The chemical composition of refined lead is predominantly pure lead (Pb), often exceeding 99.9% purity, with trace amounts of elements such as antimony, arsenic, and sulfur minimized through refining processes. The extraction and refining sequence transforms galena's sulfide composition into nearly elemental lead, crucial for industrial applications requiring high-purity lead.
Mining and Extraction Processes
Galena, the primary ore of lead, undergoes mining through both underground and open-pit methods, followed by crushing and froth flotation to concentrate the lead sulfide minerals. Refined lead is produced by smelting the concentrated galena ore at high temperatures, separating lead from sulfur and impurities through pyrometallurgical processes such as roasting and reduction. Electrolytic refining or fire refining further purifies the lead, achieving high purity levels suitable for industrial applications.
Refining Techniques: From Galena to Pure Lead
Refining lead from galena primarily involves roasting and smelting processes that convert lead sulfide (PbS) into metallic lead. Roasting oxidizes galena to lead oxide, which is then reduced in a blast furnace with carbon to yield impure lead, followed by cupellation or electrorefining techniques to achieve high-purity refined lead. These refining methods ensure removal of impurities such as silver, arsenic, and antimony, producing lead suitable for industrial applications like battery manufacturing and radiation shielding.
Physical Properties Comparison: Appearance, Density, and Hardness
Galena, a naturally occurring lead ore, exhibits a metallic gray appearance with a cubic crystal structure, while refined lead is characterized by a duller, matte finish due to impurities being removed. The density of galena averages around 7.4 g/cm3, slightly lower than refined lead, which has a higher density of approximately 11.34 g/cm3, reflecting its purer composition. In terms of hardness, galena scores lower on the Mohs scale at about 2.5, whereas refined lead is softer, ranked around 1.5, highlighting differences in their structural integrity and usability.
Industrial Uses: Galena vs Refined Lead
Galena, primarily composed of lead sulfide, serves as the main ore for extracting refined lead used in industrial applications such as batteries, radiation shielding, and ammunition. Refined lead, processed to high purity levels, offers superior durability, corrosion resistance, and malleability essential for manufacturing lead-acid batteries, cable sheathing, and construction materials. While galena itself is rarely used directly, its role as the raw mineral source is critical for producing the versatile refined lead employed across various industrial sectors.
Environmental Impact of Galena and Lead Refining
Galena (PbS), as the primary ore of lead, contains sulfur and trace heavy metals, which pose significant environmental risks during mining through acid mine drainage and toxic metal leaching. The refining process of galena, involving roasting and smelting, releases sulfur dioxide emissions contributing to air pollution and acid rain, in addition to hazardous slag waste management challenges. Refined lead production consumes substantial energy and requires stringent waste treatment to mitigate soil and water contamination from lead particulates and residues.
Economic Value: Ore vs Refined Metal
Galena, the primary ore of lead, holds significant economic value due to its high lead content and ease of extraction, but its market price is lower compared to refined lead. Refined lead commands a higher economic value because it undergoes processing to remove impurities, resulting in a purer metal suitable for industrial applications like batteries and radiation shielding. The price differential between raw galena and refined lead reflects the added cost and value of smelting, refining, and quality assurance in metal production.
Safety Considerations and Toxicity Risks
Galena (PbS) presents significant safety hazards due to lead and sulfur exposure, requiring careful handling and protective measures to prevent lead poisoning and environmental contamination. Refined lead, while purer and more stable, still poses toxicity risks through inhalation or ingestion of dust and fumes, making strict occupational safety protocols essential. Both materials demand vigilant monitoring to minimize lead accumulation in workers and surrounding ecosystems, emphasizing the importance of proper ventilation and personal protective equipment.
Galena vs Refined lead Infographic
 materialdif.com
materialdif.com