Lead battery plates are composed of lead dioxide and sponge lead, providing high energy density and excellent rechargeability, ideal for automotive and stationary power applications. Nickel-cadmium battery plates, made from nickel oxide hydroxide and cadmium, offer superior cycle life and performance in extreme temperatures but contain toxic cadmium, requiring careful disposal. Lead plates excel in cost-effectiveness and robustness, while nickel-cadmium plates are preferred for specialized uses demanding rapid charging and longevity.
Table of Comparison
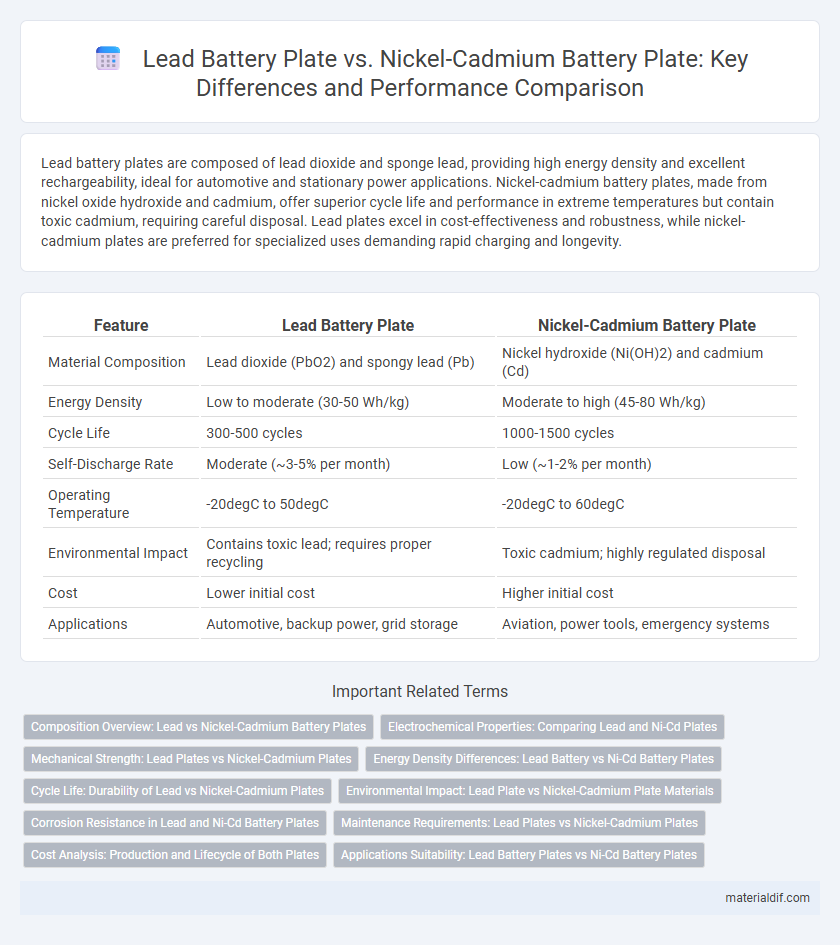
| Feature | Lead Battery Plate | Nickel-Cadmium Battery Plate |
|---|---|---|
| Material Composition | Lead dioxide (PbO2) and spongy lead (Pb) | Nickel hydroxide (Ni(OH)2) and cadmium (Cd) |
| Energy Density | Low to moderate (30-50 Wh/kg) | Moderate to high (45-80 Wh/kg) |
| Cycle Life | 300-500 cycles | 1000-1500 cycles |
| Self-Discharge Rate | Moderate (~3-5% per month) | Low (~1-2% per month) |
| Operating Temperature | -20degC to 50degC | -20degC to 60degC |
| Environmental Impact | Contains toxic lead; requires proper recycling | Toxic cadmium; highly regulated disposal |
| Cost | Lower initial cost | Higher initial cost |
| Applications | Automotive, backup power, grid storage | Aviation, power tools, emergency systems |
Composition Overview: Lead vs Nickel-Cadmium Battery Plates
Lead battery plates primarily consist of lead dioxide (PbO2) on the positive plate and spongy lead (Pb) on the negative plate, enabling efficient electrochemical reactions for energy storage. Nickel-cadmium (Ni-Cd) battery plates incorporate nickel oxide hydroxide (NiOOH) on the positive plate and cadmium (Cd) on the negative plate, offering superior cycle life and better performance in low-temperature environments. The distinct chemical compositions influence their charge-discharge characteristics, durability, and environmental impact, with lead plates being heavier and more prone to sulfation while nickel-cadmium plates resist memory effect and provide higher discharge rates.
Electrochemical Properties: Comparing Lead and Ni-Cd Plates
Lead battery plates exhibit high corrosion resistance and maintain stable electrochemical performance under varying load conditions, making them efficient for deep-cycle applications. Nickel-cadmium (Ni-Cd) plates demonstrate superior charge-discharge efficiency and faster reaction kinetics due to their unique cadmium hydroxide and nickel oxide hydroxide chemistry. The distinct electrode materials result in lead plates offering higher energy density, while Ni-Cd plates provide longer cycle life and better tolerance to overcharging.
Mechanical Strength: Lead Plates vs Nickel-Cadmium Plates
Lead battery plates exhibit higher mechanical strength and robustness compared to nickel-cadmium battery plates, making them more resistant to physical damage and deformation during heavy use. Nickel-cadmium plates, while lighter, tend to have lower structural integrity, which can result in increased susceptibility to cracking under stress or vibration. The superior mechanical durability of lead plates contributes to longer battery lifespan and enhanced performance in applications requiring frequent cycling or harsh operating conditions.
Energy Density Differences: Lead Battery vs Ni-Cd Battery Plates
Lead battery plates typically have lower energy density compared to nickel-cadmium (Ni-Cd) battery plates, which affects overall battery performance and weight. Ni-Cd battery plates offer higher energy density, enabling lighter and more compact designs ideal for portable applications. The energy density difference between lead and Ni-Cd plates directly influences the choice of battery technology in terms of efficiency and application-specific requirements.
Cycle Life: Durability of Lead vs Nickel-Cadmium Plates
Lead battery plates typically offer moderate cycle life, often ranging between 300 to 500 cycles before significant capacity loss occurs, making them suitable for applications where cost-effectiveness and moderate durability are key. Nickel-cadmium battery plates exhibit superior cycle life, with the ability to endure over 1,000 cycles due to their robust electrode structure and resistance to corrosion, thus providing enhanced longevity in high-drain or frequent recharge applications. The difference in cycle life durability is primarily attributed to the chemical stability and mechanical strength of nickel-cadmium plates compared to the more susceptible lead plates.
Environmental Impact: Lead Plate vs Nickel-Cadmium Plate Materials
Lead battery plates contain toxic lead compounds that pose significant environmental hazards during disposal and recycling, contributing to soil and water contamination. Nickel-cadmium battery plates incorporate cadmium, a highly toxic heavy metal, which presents severe ecological risks and challenges in waste management processes. Both materials require specialized handling and recycling technologies to mitigate their environmental impact effectively.
Corrosion Resistance in Lead and Ni-Cd Battery Plates
Lead battery plates exhibit superior corrosion resistance due to the formation of a stable lead sulfate layer during discharge, which protects the plate from further degradation. Nickel-cadmium battery plates, while resistant to corrosion in alkaline environments, can suffer from cadmium corrosion and oxidation over extended cycles. The inherent material properties of lead provide enhanced durability in acidic electrolytes compared to the alkaline conditions favored by Ni-Cd cells.
Maintenance Requirements: Lead Plates vs Nickel-Cadmium Plates
Lead battery plates require regular maintenance to prevent sulfation and corrosion, often involving water top-ups and ensuring proper charge levels. Nickel-cadmium battery plates demand less frequent maintenance due to their resistance to memory effect and corrosion, allowing for longer intervals between servicing. Proper maintenance of lead plates is critical to extend battery life, whereas nickel-cadmium plates benefit from a more robust and low-maintenance design.
Cost Analysis: Production and Lifecycle of Both Plates
Lead battery plates offer lower production costs due to inexpensive raw materials and simpler manufacturing processes, whereas nickel-cadmium battery plates incur higher costs driven by expensive nickel and cadmium metals and complex fabrication techniques. The lifecycle cost of lead plates benefits from widespread recycling infrastructure, reducing raw material expenses and environmental fees, in contrast to nickel-cadmium plates, which face higher disposal and regulatory compliance costs due to toxic cadmium content. While nickel-cadmium plates provide superior cycle life and performance under extreme conditions, the overall economic advantage often favors lead plates for applications prioritizing cost efficiency.
Applications Suitability: Lead Battery Plates vs Ni-Cd Battery Plates
Lead battery plates offer superior performance in high-current applications such as automotive starter batteries and backup power systems due to their robustness and cost-effectiveness. Ni-Cd battery plates excel in environments demanding long cycle life and reliable performance under extreme temperatures, making them ideal for aerospace, military, and portable power tools. The choice between lead and Ni-Cd battery plates hinges on application-specific requirements like energy density, environmental conditions, and budget constraints.
Lead battery plate vs Nickel-cadmium battery plate Infographic
 materialdif.com
materialdif.com