Lump graphite consists of natural, coarse flakes offering high purity and excellent conductivity, making it ideal for industrial applications like batteries and refractory materials. Fine graphite, with its smaller particle size and higher surface area, provides superior lubrication and enhanced bonding properties for use in lubricants and composites. Choosing between lump and fine graphite depends on the specific industrial requirements such as conductivity, purity, and mechanical performance.
Table of Comparison
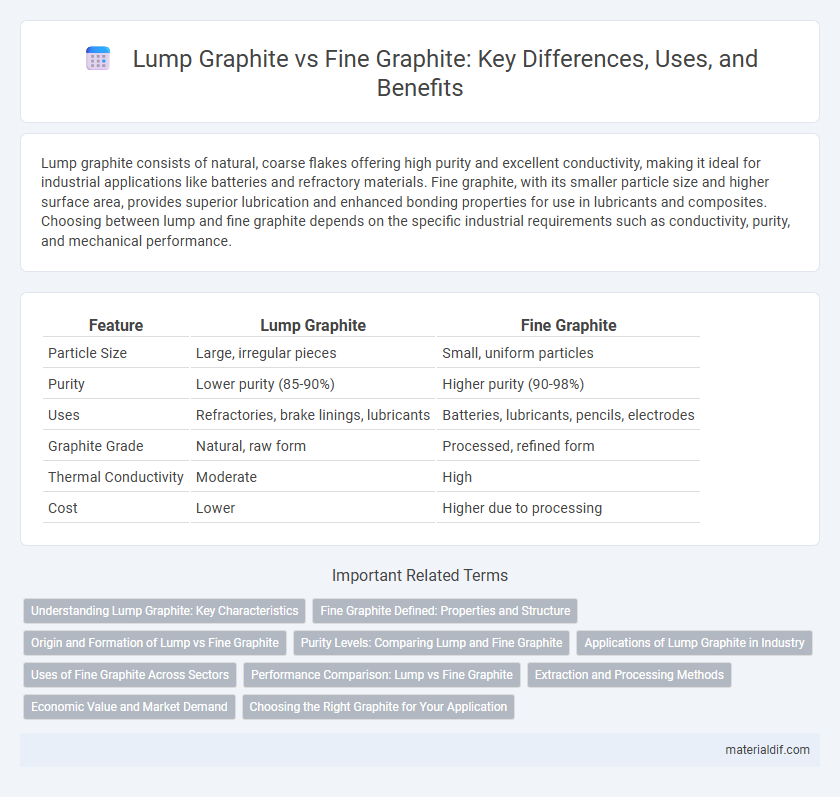
| Feature | Lump Graphite | Fine Graphite |
|---|---|---|
| Particle Size | Large, irregular pieces | Small, uniform particles |
| Purity | Lower purity (85-90%) | Higher purity (90-98%) |
| Uses | Refractories, brake linings, lubricants | Batteries, lubricants, pencils, electrodes |
| Graphite Grade | Natural, raw form | Processed, refined form |
| Thermal Conductivity | Moderate | High |
| Cost | Lower | Higher due to processing |
Understanding Lump Graphite: Key Characteristics
Lump graphite is a naturally occurring form of graphite characterized by its large, irregular chunks with a high carbon content typically exceeding 85%. Its coarse texture and low ash content make it ideal for foundry applications and refractory materials, offering excellent thermal conductivity and resistance to chemical corrosion. The physical properties of lump graphite, such as high purity and structural integrity, distinguish it from fine graphite, which is more processed and uniform.
Fine Graphite Defined: Properties and Structure
Fine graphite is characterized by smaller, more uniform particle size compared to lump graphite, enhancing its packing density and surface area. Its structure consists of tightly packed, flaky particles with high purity and excellent thermal and electrical conductivity, making it ideal for applications requiring precision and consistency. Fine graphite's unique combination of high carbon content and minimal impurities improves mechanical strength and resistance to oxidation.
Origin and Formation of Lump vs Fine Graphite
Lump graphite forms naturally as large, crystalline flakes within metamorphic rocks such as schists and gneisses, originating from the metamorphism of carbon-rich sediments under high temperature and pressure conditions. Fine graphite, on the other hand, typically results from the breakdown of lump graphite or secondary geological processes, appearing as smaller, more dispersed particles often associated with hydrothermal alteration or weathering. The distinct formation environments influence their morphology, purity, and industrial applications in fields like refractory manufacturing and battery technology.
Purity Levels: Comparing Lump and Fine Graphite
Lump graphite typically exhibits higher purity levels with carbon content often exceeding 95%, making it ideal for applications requiring superior conductivity and chemical resistance. Fine graphite, while containing slightly lower purity around 80-90% carbon, offers greater surface area and reactivity, suited for processes needing enhanced graphite dispersion. Purity distinctions between lump and fine graphite significantly influence their performance in industries such as battery anodes, refractories, and lubricants.
Applications of Lump Graphite in Industry
Lump graphite is widely used in steel manufacturing for its excellent lubricant properties and ability to improve carbon content and structure during production. Industries utilize lump graphite in refractory materials, electrodes for electric arc furnaces, and brake linings due to its high thermal resistance and electrical conductivity. Its natural purity and flaky structure make it ideal for foundry facings and lubricants in heavy machinery applications.
Uses of Fine Graphite Across Sectors
Fine graphite is extensively used in batteries, lubricants, and conductive materials due to its small particle size and high purity. Its applications span the automotive industry for brake linings, the electronics sector for conductive coatings, and the manufacturing of pencils and refractory materials. Fine graphite's unique properties enhance performance in energy storage devices and industrial components requiring excellent thermal and electrical conductivity.
Performance Comparison: Lump vs Fine Graphite
Lump graphite exhibits higher purity and better electrical conductivity compared to fine graphite, making it ideal for applications requiring superior performance, such as in batteries and lubricants. Fine graphite, with its smaller particle size, offers enhanced surface area and reactivity, benefiting processes like refractory manufacturing and conductive coatings. Performance differences between lump and fine graphite impact thermal stability, compression strength, and electrical conductivity, influencing their suitability across industrial uses.
Extraction and Processing Methods
Lump graphite is typically extracted through traditional mining techniques involving open-pit or underground mining, followed by crushing and sizing to separate large, natural graphite flakes. Fine graphite, often sourced from both natural deposits and synthetic production, requires more intricate processing such as flotation and chemical treatments to achieve higher purity and finer particle size. Advanced purification methods like acid leaching and thermal purification are frequently applied to fine graphite to meet industrial specifications for batteries and electronics.
Economic Value and Market Demand
Lump graphite commands a higher economic value due to its purity and larger flake size, making it more desirable for high-tech applications such as batteries and refractories. Fine graphite, while lower in price, maintains steady market demand driven by its suitability for applications like lubricants and brake linings. Market trends indicate increasing preference for lump graphite amid rising demand in electric vehicle and renewable energy sectors.
Choosing the Right Graphite for Your Application
Lump graphite offers higher purity and larger crystal sizes, making it ideal for applications requiring excellent electrical conductivity and high thermal resistance, such as electrodes and refractory materials. Fine graphite, with its smaller particle size and greater surface area, is better suited for lubricants, batteries, and paints where uniform dispersion and enhanced reactivity are critical. Selecting the right graphite depends on evaluating the specific performance needs, particle size, and purity requirements of your industrial or chemical process.
Lump Graphite vs Fine Graphite Infographic
 materialdif.com
materialdif.com