Primary graphite is a naturally occurring form extracted directly from mining operations, valued for its high purity and crystalline structure ideal for industrial applications. Secondary graphite is produced through recycling or reprocessing of graphite-containing materials, offering a more sustainable and cost-effective alternative. Both types serve distinct roles in the graphite industry, with primary graphite favored for high-performance uses and secondary graphite supporting environmental and economic goals.
Table of Comparison
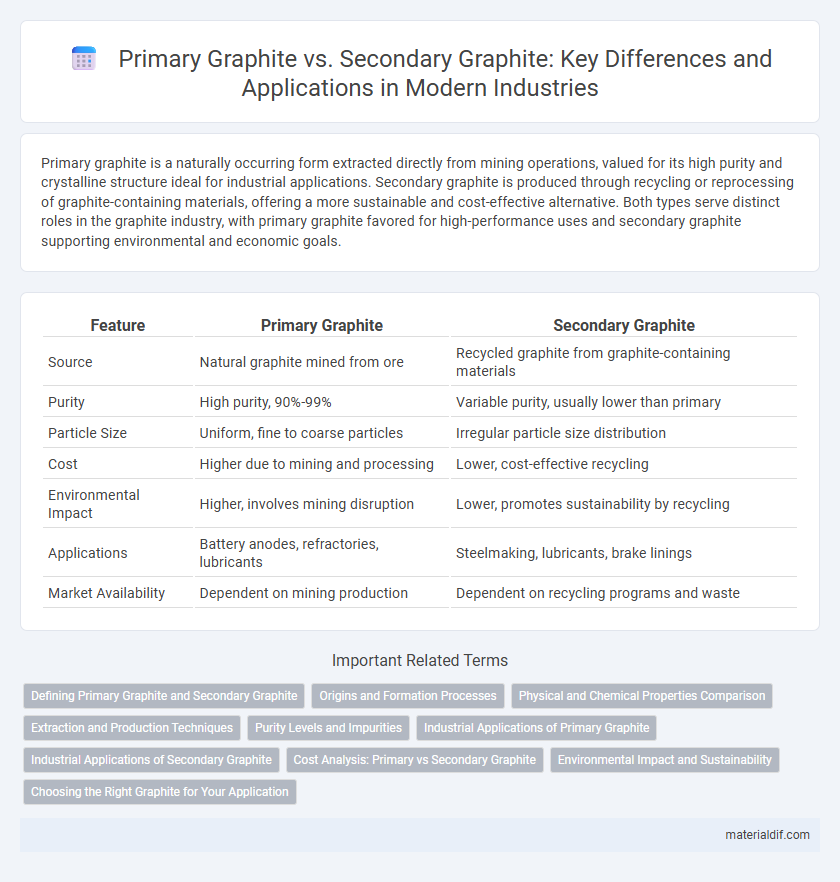
| Feature | Primary Graphite | Secondary Graphite |
|---|---|---|
| Source | Natural graphite mined from ore | Recycled graphite from graphite-containing materials |
| Purity | High purity, 90%-99% | Variable purity, usually lower than primary |
| Particle Size | Uniform, fine to coarse particles | Irregular particle size distribution |
| Cost | Higher due to mining and processing | Lower, cost-effective recycling |
| Environmental Impact | Higher, involves mining disruption | Lower, promotes sustainability by recycling |
| Applications | Battery anodes, refractories, lubricants | Steelmaking, lubricants, brake linings |
| Market Availability | Dependent on mining production | Dependent on recycling programs and waste |
Defining Primary Graphite and Secondary Graphite
Primary graphite forms naturally through metamorphic processes in crystalline rocks, exhibiting well-defined flake or crystalline structures commonly found in schists or gneisses. Secondary graphite originates from the chemical decomposition of carbonaceous material during sedimentary diagenesis, resulting in fine-grained, amorphous, or microcrystalline graphite deposits typically associated with coal seams or carbon-rich shales. The distinction between primary and secondary graphite is critical for applications requiring specific physical properties and purity levels.
Origins and Formation Processes
Primary graphite originates from high-grade metamorphic processes where carbon-rich sediments undergo intense heat and pressure over millions of years, resulting in crystalline graphite deposits often found in schist and gneiss formations. Secondary graphite forms through the alteration of organic material and secondary mineral processes, typically in sedimentary environments where hydrothermal fluids or weathering lead to the recrystallization of carbon. The distinct origins and formation mechanisms influence their purity, crystal structure, and applications in industries such as battery manufacturing and refractories.
Physical and Chemical Properties Comparison
Primary graphite, naturally occurring in crystalline form, exhibits higher purity with a carbon content typically above 90%, characterized by well-ordered crystalline layers that offer excellent electrical conductivity and thermal stability. Secondary graphite, derived from the reprocessing of carbon-rich materials, often contains impurities and exhibits a more amorphous structure, resulting in lower conductivity and reduced chemical resistance compared to primary graphite. The physical hardness and density of primary graphite tend to be greater, while secondary graphite's properties vary significantly based on the source material and processing methods.
Extraction and Production Techniques
Primary graphite is extracted from natural graphite deposits through open-pit or underground mining, followed by flotation and refining processes to enhance purity and flake size. Secondary graphite, derived from recycled graphite materials, is produced using mechanical grinding, purification, and thermal treatment to restore graphite quality suitable for industrial applications. Advanced production techniques for both types emphasize maintaining high crystallinity and minimizing impurities to meet the demands of electronics and battery manufacturing.
Purity Levels and Impurities
Primary graphite exhibits higher purity levels, often exceeding 90% carbon content, with minimal impurities such as silica, alumina, and iron oxides, making it ideal for applications requiring superior conductivity and thermal stability. Secondary graphite, derived from recycled or synthetic sources, typically contains lower purity, around 70-85%, with increased impurities including metallic contaminants and residual binders, affecting performance in high-precision industries. The choice between primary and secondary graphite hinges on balancing purity requirements against cost and environmental considerations.
Industrial Applications of Primary Graphite
Primary graphite, also known as natural graphite, is mined directly from metamorphic rock deposits and is prized for its high purity and crystalline structure, making it ideal for industrial applications requiring high thermal and electrical conductivity. It is extensively used in the manufacturing of batteries, refractory materials, lubricants, and graphite electrodes essential for electric arc furnace steel production. The consistent quality and large flake size of primary graphite enhance its performance in advanced industries such as aerospace, electronics, and nuclear reactors.
Industrial Applications of Secondary Graphite
Secondary graphite, derived from the recycling of graphite products, plays a crucial role in industrial applications by providing a sustainable and cost-effective alternative to primary graphite. It is extensively used in manufacturing electrodes for electric arc furnaces, lubricants, and refractory materials due to its high purity and consistent performance. The demand for secondary graphite is growing rapidly in battery production, especially for lithium-ion batteries in electric vehicles, where high-quality recycled graphite enhances energy efficiency and reduces environmental impact.
Cost Analysis: Primary vs Secondary Graphite
Primary graphite, extracted through mining natural graphite deposits, generally incurs higher production costs due to extensive mining operations and processing requirements. Secondary graphite, produced by recycling graphite-containing materials, offers a cost-efficient alternative by reducing raw material expenses and energy consumption. Cost analysis reveals that secondary graphite's lower operational costs and environmental benefits make it increasingly attractive for industrial applications, despite potential purity variations compared to primary graphite.
Environmental Impact and Sustainability
Primary graphite, extracted through mining natural graphite deposits, often involves significant land disturbance and energy-intensive processes, leading to higher carbon emissions and habitat disruption. Secondary graphite, produced by recycling graphite-containing materials such as spent lithium-ion batteries and industrial waste, significantly reduces environmental impact by minimizing raw material extraction and lowering energy consumption. Emphasizing secondary graphite enhances sustainability efforts by promoting resource circularity and reducing reliance on finite natural graphite reserves.
Choosing the Right Graphite for Your Application
Primary graphite, sourced directly from natural graphite deposits, offers superior purity and crystallinity, making it ideal for high-performance applications such as batteries and lubricants. Secondary graphite, typically derived from recycled materials or industrial by-products, provides a cost-effective alternative with slightly lower structural integrity, suitable for less demanding uses like refractory products. Selecting the right graphite depends on application requirements including thermal conductivity, electrical performance, and budget constraints, ensuring optimal efficiency and material compatibility.
Primary Graphite vs Secondary Graphite Infographic
 materialdif.com
materialdif.com