Synthetic graphite offers superior purity and consistent particle size compared to carbon black, making it ideal for high-performance battery anodes and industrial applications. Carbon black excels in reinforcing rubber and enhancing electrical conductivity but lacks the structural uniformity and thermal stability of synthetic graphite. Choosing between the two materials depends on specific requirements such as conductivity, mechanical strength, and chemical resistance.
Table of Comparison
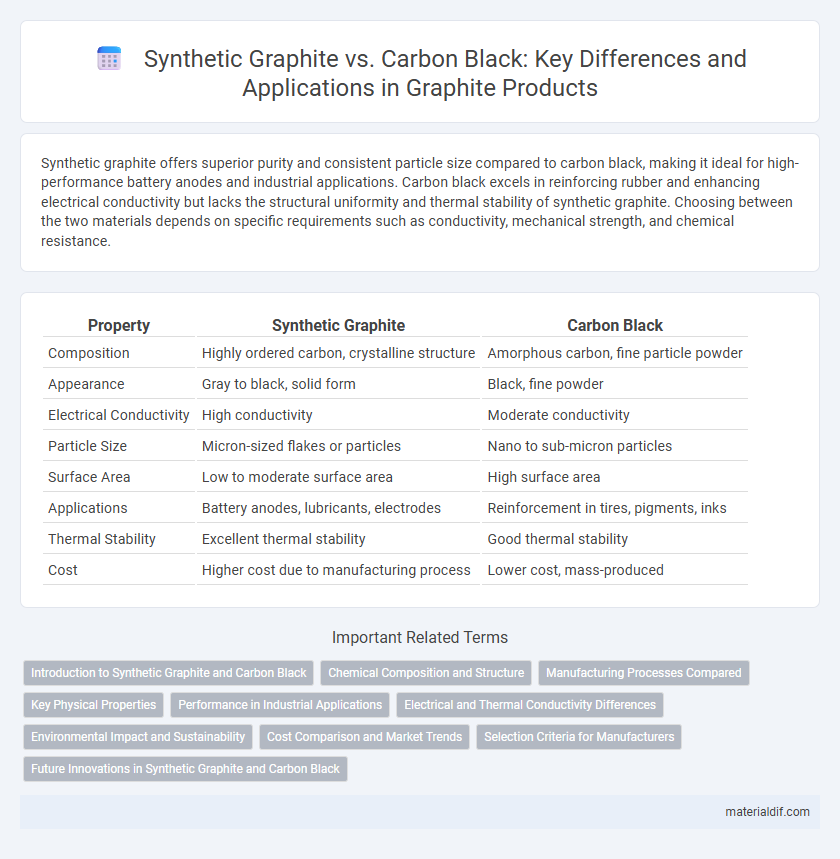
| Property | Synthetic Graphite | Carbon Black |
|---|---|---|
| Composition | Highly ordered carbon, crystalline structure | Amorphous carbon, fine particle powder |
| Appearance | Gray to black, solid form | Black, fine powder |
| Electrical Conductivity | High conductivity | Moderate conductivity |
| Particle Size | Micron-sized flakes or particles | Nano to sub-micron particles |
| Surface Area | Low to moderate surface area | High surface area |
| Applications | Battery anodes, lubricants, electrodes | Reinforcement in tires, pigments, inks |
| Thermal Stability | Excellent thermal stability | Good thermal stability |
| Cost | Higher cost due to manufacturing process | Lower cost, mass-produced |
Introduction to Synthetic Graphite and Carbon Black
Synthetic graphite is a highly crystalline form of carbon produced through the high-temperature treatment of carbon-rich materials, offering superior electrical conductivity and thermal stability compared to carbon black. Carbon black, derived from the incomplete combustion of hydrocarbons, consists of fine particles primarily used as a reinforcing filler in tires and pigments in inks and coatings. The unique structural properties of synthetic graphite make it ideal for applications such as battery anodes and electrodes, while carbon black's high surface area and dispersibility suit applications requiring pigmentation and reinforcement.
Chemical Composition and Structure
Synthetic graphite consists primarily of high-purity carbon arranged in a highly ordered crystalline structure, which enhances its electrical conductivity and thermal stability. Carbon black, in contrast, features an amorphous carbon structure with a high surface area and contains various impurities, affecting its reactivity and dispersibility. The chemical composition of synthetic graphite is more consistent, typically exceeding 99% carbon, while carbon black contains lower carbon content along with traces of residual hydrocarbons and ash.
Manufacturing Processes Compared
Synthetic graphite is produced through high-temperature treatment of petroleum coke and pitch in electric furnaces, enabling precise control over purity and crystallinity. Carbon black manufacturing involves incomplete combustion or thermal decomposition of hydrocarbons, resulting in fine particulate matter with varying surface areas but less structural order than synthetic graphite. The controlled graphitization process in synthetic graphite offers superior electrical conductivity and mechanical strength compared to the amorphous carbon structure of carbon black.
Key Physical Properties
Synthetic graphite exhibits higher purity and crystallinity compared to carbon black, resulting in superior electrical conductivity and thermal stability. Its structured, layered morphology provides enhanced mechanical strength and resistance to oxidation, whereas carbon black has a more amorphous structure with lower density and surface area variations. These key physical differences make synthetic graphite preferable for high-performance applications like batteries and electrodes, while carbon black is commonly used as a reinforcing agent and pigment.
Performance in Industrial Applications
Synthetic graphite offers superior electrical conductivity and thermal stability compared to carbon black, making it ideal for high-performance applications such as batteries, fuel cells, and electric motors. Its uniform particle size and high purity enhance mechanical strength and durability in industrial lubricants and refractory materials. Carbon black, while cost-effective and excellent for pigmentation and reinforcement in rubber products, lacks the consistent conductivity and thermal resilience required for advanced industrial uses.
Electrical and Thermal Conductivity Differences
Synthetic graphite exhibits significantly higher electrical and thermal conductivity compared to carbon black due to its highly ordered crystalline structure. Carbon black, composed of amorphous carbon particles, offers lower conductivity, making it suitable primarily for applications requiring partial conductivity and reinforcement rather than efficient heat or electrical transfer. The improved conductivity of synthetic graphite makes it ideal for advanced battery electrodes, conductive coatings, and thermal management systems.
Environmental Impact and Sustainability
Synthetic graphite production typically requires higher energy consumption and emits more greenhouse gases compared to carbon black manufacturing, impacting its environmental footprint. Carbon black, often derived as a byproduct of fossil fuel combustion, poses recycling challenges but generally has lower energy demands during production. Sustainable advancements focus on improving energy efficiency for synthetic graphite and developing bio-based or recycled carbon black to reduce environmental impact.
Cost Comparison and Market Trends
Synthetic graphite commands a higher price than carbon black due to its complex manufacturing process and superior electrical conductivity. Market trends indicate increasing demand for synthetic graphite in battery anodes, driving price premiums despite cost challenges. Carbon black remains cost-effective for tire and rubber applications, maintaining steady market share amid fluctuating raw material costs.
Selection Criteria for Manufacturers
Manufacturers prioritize purity, particle size distribution, and electrical conductivity when selecting synthetic graphite over carbon black for industrial applications. Synthetic graphite offers superior structural uniformity and thermal stability, essential for battery anodes and lubricants, whereas carbon black excels in pigmentation and reinforcement due to its high surface area. Cost-effectiveness and processing compatibility also influence the decision, balancing performance requirements with production efficiency.
Future Innovations in Synthetic Graphite and Carbon Black
Future innovations in synthetic graphite focus on enhancing battery performance, increasing energy density, and improving cycle life for electric vehicles and energy storage systems. Advancements in carbon black production emphasize sustainability, including bio-based feedstocks and lower carbon emissions during manufacturing. Both materials are pivotal in evolving industries, with research targeting nanostructured synthetic graphite and high-purity carbon black to meet growing demands.
Synthetic Graphite vs Carbon Black Infographic
 materialdif.com
materialdif.com