High-purity graphite offers superior electrical conductivity and thermal stability compared to regular graphite, making it ideal for advanced industrial applications. Its low impurity levels enhance performance in battery anodes, fuel cells, and high-temperature environments. Regular graphite, while more affordable, contains higher impurities that can affect durability and efficiency in high-demand uses.
Table of Comparison
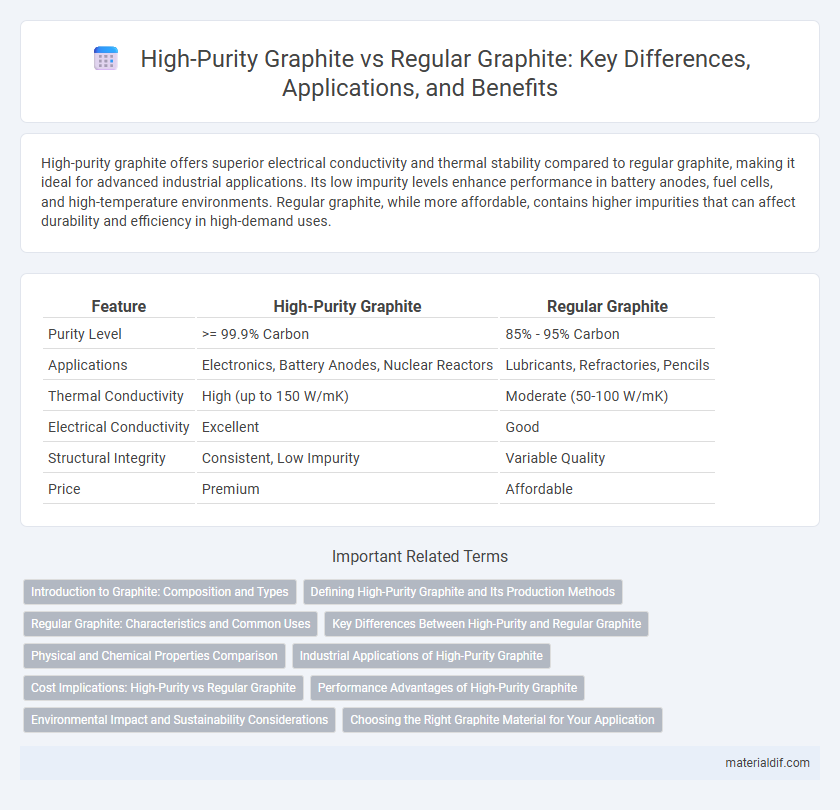
| Feature | High-Purity Graphite | Regular Graphite |
|---|---|---|
| Purity Level | >= 99.9% Carbon | 85% - 95% Carbon |
| Applications | Electronics, Battery Anodes, Nuclear Reactors | Lubricants, Refractories, Pencils |
| Thermal Conductivity | High (up to 150 W/mK) | Moderate (50-100 W/mK) |
| Electrical Conductivity | Excellent | Good |
| Structural Integrity | Consistent, Low Impurity | Variable Quality |
| Price | Premium | Affordable |
Introduction to Graphite: Composition and Types
High-purity graphite is characterized by a carbon content exceeding 99.9%, making it ideal for specialized applications requiring exceptional thermal and electrical conductivity. Regular graphite typically contains impurities such as ash and metallic elements, which can affect its performance in critical industrial processes. The primary types of graphite are natural and synthetic, each varying in purity, crystalline structure, and suitability for different technological uses.
Defining High-Purity Graphite and Its Production Methods
High-purity graphite contains a carbon content exceeding 99.95%, achieved through advanced purification techniques such as chemical vapor deposition, high-temperature thermal treatment, and acid leaching, which remove impurities like metallic and non-metallic contaminants. This specialized graphite is essential for applications requiring exceptional electrical conductivity, thermal stability, and chemical inertness, distinguishing it from regular graphite that typically contains higher impurity levels and exhibits lower performance in critical industrial uses. Production methods prioritize purity enhancement by controlling raw material selection and employing multi-stage refinement processes to ensure uniform particle size and structural consistency.
Regular Graphite: Characteristics and Common Uses
Regular graphite, characterized by lower purity levels typically around 85-95%, contains impurities such as ash, sulfur, and metallic elements that affect its physical and chemical properties. It exhibits good thermal and electrical conductivity, moderate strength, and is commonly used in applications like battery electrodes, brake linings, lubricants, and steelmaking refractory materials. Due to its cost-effectiveness and adequate performance, regular graphite remains a preferred choice for industrial sectors requiring bulk material with consistent quality but not ultra-high purity.
Key Differences Between High-Purity and Regular Graphite
High-purity graphite contains carbon levels exceeding 99.99%, whereas regular graphite typically ranges between 85% to 95% carbon content, impacting their performance in advanced applications. The superior purity of high-purity graphite results in enhanced electrical conductivity, thermal stability, and chemical resistance compared to regular graphite. These characteristics make high-purity graphite essential for demanding industries such as semiconductors, aerospace, and nuclear reactors, while regular graphite often suffices for general industrial uses like lubricants and batteries.
Physical and Chemical Properties Comparison
High-purity graphite exhibits superior physical properties such as higher density, enhanced thermal conductivity, and increased electrical conductivity compared to regular graphite. Chemically, high-purity graphite contains significantly lower levels of impurities like sulfur, iron, and ash content, resulting in improved corrosion resistance and chemical stability under extreme conditions. These differences make high-purity graphite essential for applications requiring consistent performance in aerospace, semiconductors, and nuclear reactors.
Industrial Applications of High-Purity Graphite
High-purity graphite, characterized by impurity levels below 50 ppm, is essential in semiconductor manufacturing, lithium-ion battery anodes, and nuclear reactors due to its exceptional electrical conductivity and thermal stability. Its superior purity ensures minimal contamination in sensitive industrial processes, enhancing product reliability and performance. Regular graphite, with higher impurity content, is typically restricted to less demanding applications such as brake linings and lubricants.
Cost Implications: High-Purity vs Regular Graphite
High-purity graphite commands significantly higher costs compared to regular graphite due to its extensive refining process and superior chemical stability, critical for applications in electronics and aerospace. Regular graphite, while cheaper, contains more impurities that limit its use to less demanding industrial purposes such as brake linings and lubricants. Cost considerations often dictate material selection, balancing between performance requirements and budget constraints in manufacturing.
Performance Advantages of High-Purity Graphite
High-purity graphite exhibits superior electrical conductivity and thermal stability compared to regular graphite, making it ideal for advanced electronic and thermal management applications. Its minimal impurity levels reduce the risk of contamination, enhancing performance consistency in high-precision industries such as semiconductor manufacturing and aerospace. The enhanced durability and chemical resistance of high-purity graphite also extend the lifespan of components under extreme operational conditions.
Environmental Impact and Sustainability Considerations
High-purity graphite significantly reduces environmental impact compared to regular graphite by producing fewer impurities during mining and refining processes, leading to less toxic waste and lower energy consumption in industrial applications. Its enhanced sustainability is driven by longer material lifespan and improved recyclability, minimizing resource extraction and ecological disruption over time. Selecting high-purity graphite supports cleaner production methods and aligns with green manufacturing standards essential for industries prioritizing carbon footprint reduction.
Choosing the Right Graphite Material for Your Application
High-purity graphite offers superior electrical conductivity, chemical resistance, and thermal stability compared to regular graphite, making it ideal for applications requiring minimal impurities such as semiconductor manufacturing and aerospace components. Regular graphite, though more cost-effective, contains higher levels of impurities that can affect performance in sensitive environments but remains suitable for general industrial use, including lubricants and refractory materials. Selecting the right graphite material depends on balancing purity levels with application-specific requirements for conductivity, durability, and thermal management.
High-purity Graphite vs Regular Graphite Infographic
 materialdif.com
materialdif.com