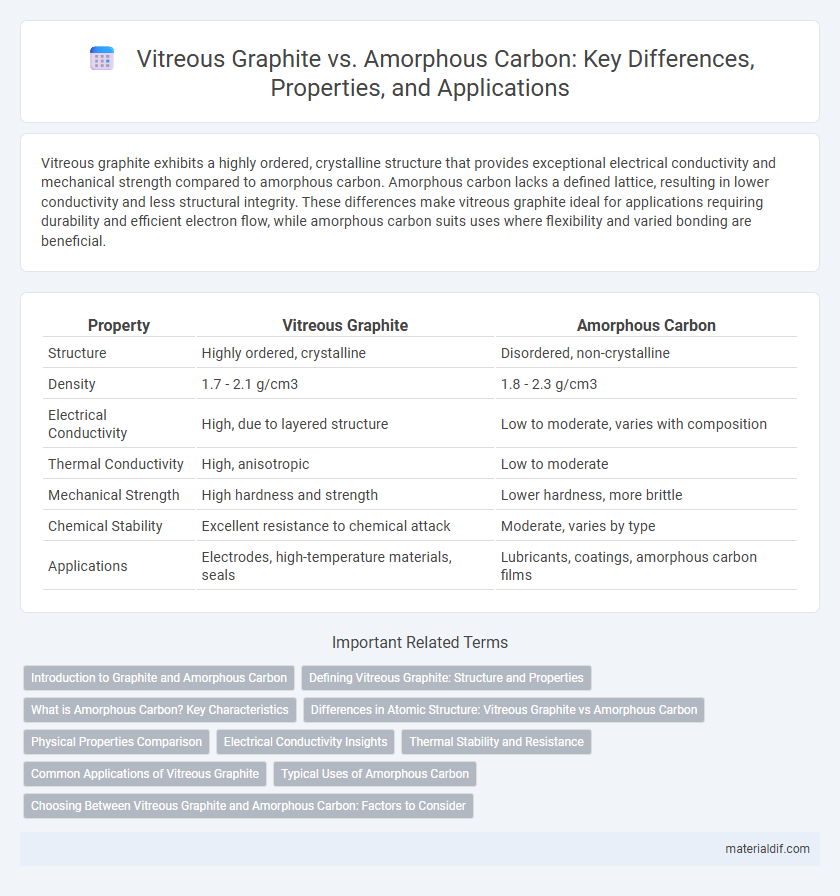
Vitreous graphite exhibits a highly ordered, crystalline structure that provides exceptional electrical conductivity and mechanical strength compared to amorphous carbon. Amorphous carbon lacks a defined lattice, resulting in lower conductivity and less structural integrity. These differences make vitreous graphite ideal for applications requiring durability and efficient electron flow, while amorphous carbon suits uses where flexibility and varied bonding are beneficial.
Table of Comparison
| Property | Vitreous Graphite | Amorphous Carbon |
|---|---|---|
| Structure | Highly ordered, crystalline | Disordered, non-crystalline |
| Density | 1.7 - 2.1 g/cm3 | 1.8 - 2.3 g/cm3 |
| Electrical Conductivity | High, due to layered structure | Low to moderate, varies with composition |
| Thermal Conductivity | High, anisotropic | Low to moderate |
| Mechanical Strength | High hardness and strength | Lower hardness, more brittle |
| Chemical Stability | Excellent resistance to chemical attack | Moderate, varies by type |
| Applications | Electrodes, high-temperature materials, seals | Lubricants, coatings, amorphous carbon films |
Introduction to Graphite and Amorphous Carbon
Graphite is a crystalline form of carbon characterized by its organized hexagonal layers, providing high electrical conductivity and thermal resistance. Vitreous graphite, a type of synthetic graphite, exhibits a dense, glassy texture with enhanced strength and chemical stability compared to amorphous carbon. Amorphous carbon lacks a defined crystalline structure, resulting in lower conductivity and differs significantly in mechanical properties from graphite.
Defining Vitreous Graphite: Structure and Properties
Vitreous graphite is a form of carbon characterized by a highly ordered, crystalline structure exhibiting graphitic layers with strong covalent bonds arranged in a hexagonal lattice, resulting in excellent electrical conductivity and thermal stability. Unlike amorphous carbon, which lacks long-range order and consists of randomly arranged carbon atoms leading to lower density and inferior mechanical strength, vitreous graphite's dense, anisotropic structure imparts superior hardness and chemical resistance. Key properties of vitreous graphite include high purity, anisotropic electrical conductivity, low coefficient of thermal expansion, and resistance to chemical attack, distinguishing it significantly from the more disordered amorphous carbon materials.
What is Amorphous Carbon? Key Characteristics
Amorphous carbon is a non-crystalline form of carbon lacking the ordered lattice structure found in vitreous graphite. Key characteristics include a random atomic arrangement, high surface area, and varying electrical conductivity depending on bonding and impurities. This material is commonly used in applications requiring porous carbon, such as electrodes, adsorbents, and lubricants.
Differences in Atomic Structure: Vitreous Graphite vs Amorphous Carbon
Vitreous graphite exhibits a highly ordered atomic structure with graphene layers arranged in a turbostratic or semi-crystalline fashion, enhancing its electrical conductivity and mechanical strength. In contrast, amorphous carbon lacks long-range order, consisting of randomly bonded carbon atoms with mixed sp2 and sp3 hybridizations, resulting in lower crystallinity and variable properties. The distinct atomic arrangements in vitreous graphite and amorphous carbon determine their differing electrical, thermal, and mechanical behaviors.
Physical Properties Comparison
Vitreous graphite exhibits a highly ordered crystalline structure, resulting in superior thermal conductivity and electrical resistance compared to amorphous carbon, which has a disordered atomic arrangement. The density of vitreous graphite is typically higher, contributing to its greater mechanical strength and hardness relative to the more porous and softer amorphous carbon. Furthermore, vitreous graphite's anisotropic physical properties contrast with the isotropic nature of amorphous carbon, making it more suitable for specialized industrial applications requiring directional conductivity and rigidity.
Electrical Conductivity Insights
Vitreous graphite exhibits significantly higher electrical conductivity compared to amorphous carbon due to its well-ordered crystalline structure facilitating efficient electron mobility. The sp2 hybridization and layered arrangement in vitreous graphite create delocalized pi-electron clouds that enhance electrical conduction pathways. In contrast, amorphous carbon's disordered atomic arrangement disrupts electron flow, resulting in substantially lower electrical conductivity and increased resistivity.
Thermal Stability and Resistance
Vitreous graphite exhibits superior thermal stability compared to amorphous carbon, maintaining structural integrity at temperatures exceeding 3000degC, while amorphous carbon typically degrades around 1000degC. The resistance of vitreous graphite to thermal shock and oxidation is significantly higher due to its ordered crystalline structure and low impurity content. In contrast, amorphous carbon's disordered bonding results in lower resistance and rapid deterioration under high temperature conditions.
Common Applications of Vitreous Graphite
Vitreous graphite is commonly used in high-temperature applications such as electrodes in electric arc furnaces, crucibles for metal melting, and parts in nuclear reactors due to its excellent thermal stability and electrical conductivity. Unlike amorphous carbon, which is predominantly applied in lubricants and coatings, vitreous graphite's crystalline structure enables superior mechanical strength and chemical resistance, making it ideal for industrial processes involving extreme conditions. Its use in semiconductor manufacturing and aerospace components further highlights its critical role in advanced technological applications requiring durability and precision.
Typical Uses of Amorphous Carbon
Amorphous carbon is widely used as a coating material in industrial applications due to its excellent hardness and chemical resistance. It serves as a crucial component in lubricants and batteries, enhancing wear resistance and electrical conductivity. Typical applications include protective films in automotive parts, cutting tools, and electronic devices where durability and thermal stability are essential.
Choosing Between Vitreous Graphite and Amorphous Carbon: Factors to Consider
Choosing between vitreous graphite and amorphous carbon hinges on the desired electrical conductivity and thermal stability; vitreous graphite offers superior conductivity and higher thermal tolerance due to its crystalline structure. Amorphous carbon, with its disordered atomic arrangement, provides better chemical resistance and lower density, making it ideal for lightweight, corrosion-resistant applications. Assessing mechanical strength, conductivity requirements, and environmental exposure is crucial for optimal material selection.
Vitreous Graphite vs Amorphous Carbon Infographic
 materialdif.com
materialdif.com