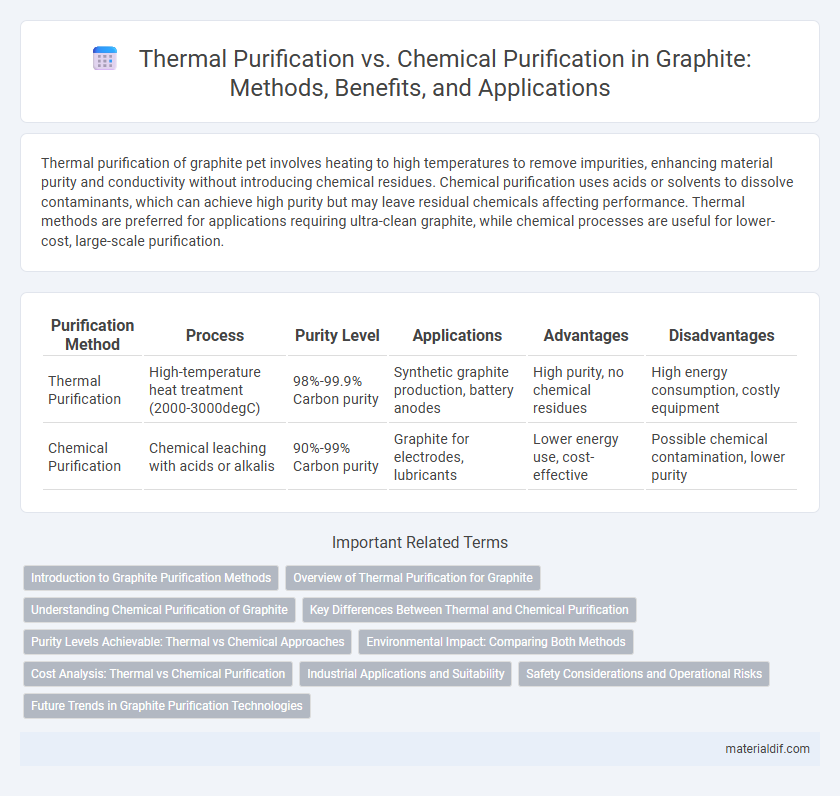
Thermal purification of graphite pet involves heating to high temperatures to remove impurities, enhancing material purity and conductivity without introducing chemical residues. Chemical purification uses acids or solvents to dissolve contaminants, which can achieve high purity but may leave residual chemicals affecting performance. Thermal methods are preferred for applications requiring ultra-clean graphite, while chemical processes are useful for lower-cost, large-scale purification.
Table of Comparison
| Purification Method | Process | Purity Level | Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Thermal Purification | High-temperature heat treatment (2000-3000degC) | 98%-99.9% Carbon purity | Synthetic graphite production, battery anodes | High purity, no chemical residues | High energy consumption, costly equipment |
| Chemical Purification | Chemical leaching with acids or alkalis | 90%-99% Carbon purity | Graphite for electrodes, lubricants | Lower energy use, cost-effective | Possible chemical contamination, lower purity |
Introduction to Graphite Purification Methods
Graphite purification methods primarily include thermal purification and chemical purification, each targeting the removal of impurities to enhance graphite quality. Thermal purification utilizes high temperatures, often above 2500degC, to vaporize volatile contaminants, resulting in ultra-high purity graphite suitable for advanced applications like batteries and electronics. Chemical purification involves acid or alkaline treatments, which dissolve metallic and non-metallic impurities, offering precise control over purity levels while maintaining graphite's structural integrity.
Overview of Thermal Purification for Graphite
Thermal purification for graphite involves heating the material to extremely high temperatures, typically above 2,500degC, to remove impurities such as ash, metallic oxides, and volatile substances. This process enhances the purity and electrical conductivity of graphite, making it ideal for high-performance applications in batteries and electronics. Compared to chemical purification, thermal purification offers a cleaner, residue-free method that preserves the graphite's structural integrity.
Understanding Chemical Purification of Graphite
Chemical purification of graphite involves using strong acids, alkalis, or oxidizing agents to remove impurities such as metal particles and non-carbon materials, enhancing its purity beyond thermal methods. This process often includes treatments with hydrofluoric acid, nitric acid, or potassium permanganate to dissolve contaminants without compromising the graphite's crystalline structure. Chemical purification results in higher-quality graphite with improved electrical conductivity and chemical stability, essential for advanced applications in batteries, electronics, and high-performance composites.
Key Differences Between Thermal and Chemical Purification
Thermal purification of graphite involves high-temperature treatment, typically above 2500degC, to remove impurities by volatilization and structural reordering, enhancing crystallinity and purity. Chemical purification uses acid or alkaline solutions to dissolve metallic and non-metallic contaminants at lower temperatures, preserving the graphite structure but potentially introducing residues. Key differences include the temperature range, impurity removal mechanisms, and impact on graphite microstructure and surface chemistry.
Purity Levels Achievable: Thermal vs Chemical Approaches
Thermal purification of graphite typically achieves purity levels exceeding 99.9%, effectively removing volatile impurities through high-temperature treatments above 2500degC. Chemical purification, involving acid leaching or oxidative methods, can attain similar purity levels but often introduces risks of structural damage and residual chemicals. Choosing between thermal and chemical approaches depends on the required purity balance against structural integrity and processing costs.
Environmental Impact: Comparing Both Methods
Thermal purification of graphite involves high-temperature processes that consume significant energy and release greenhouse gases, contributing to a larger carbon footprint compared to chemical purification. Chemical purification uses solvents and acids, posing risks of toxic waste generation and water contamination if not properly managed, but generally requires less energy. Evaluating environmental impact emphasizes the trade-off between energy consumption and chemical waste, highlighting the need for sustainable waste treatment and energy-efficient technologies in graphite purification.
Cost Analysis: Thermal vs Chemical Purification
Thermal purification of graphite generally incurs higher energy costs due to prolonged high-temperature processes reaching up to 3000degC, while chemical purification involves expenses related to hazardous reagents such as hydrofluoric acid and acid waste management. Cost analysis reveals thermal methods have lower chemical handling and environmental compliance costs but higher operational energy consumption. Chemical purification offers faster processing times and lower energy requirements but demands significant investment in chemical procurement and safety measures.
Industrial Applications and Suitability
Thermal purification of graphite is widely favored in industrial applications requiring ultra-high purity due to its efficiency in removing metallic impurities through high-temperature treatment above 2500degC, making it suitable for electronic components and nuclear reactors. Chemical purification, involving acid or alkali treatments, is more appropriate for applications demanding moderate purity levels, such as battery anodes and lubricants, due to lower cost and scalability despite less effective impurity removal. The choice between thermal and chemical purification depends on the targeted purity, application scale, and economic considerations in industries like aerospace, energy storage, and metallurgy.
Safety Considerations and Operational Risks
Thermal purification of graphite involves high-temperature treatment, posing significant fire and explosion hazards due to the release of flammable gases, necessitating stringent temperature control and proper ventilation. Chemical purification uses aggressive acids or alkalis, which present risks of chemical burns, toxic fumes, and hazardous waste disposal challenges, requiring robust handling protocols and personal protective equipment. Both methods demand comprehensive safety measures to mitigate operational risks and ensure safe processing environments.
Future Trends in Graphite Purification Technologies
Future trends in graphite purification technologies emphasize enhanced thermal purification methods that improve efficiency by targeting impurity volatilization at controlled high temperatures. Chemical purification advancements focus on eco-friendly reagents and process integration to reduce waste and energy consumption. Emerging hybrid techniques combining thermal and chemical processes offer promising pathways for producing ultra-high purity graphite required in energy storage and advanced electronics industries.
Thermal Purification vs Chemical Purification Infographic
 materialdif.com
materialdif.com