Battery-grade graphite offers superior purity and consistent electrochemical properties essential for high-performance lithium-ion batteries, whereas refractory-grade graphite is primarily used in industrial applications requiring high thermal stability and durability. The structural differences influence battery efficiency, with battery-grade graphite providing better conductivity and longer cycle life. Selecting the appropriate grade directly impacts the effectiveness and longevity of energy storage solutions.
Table of Comparison
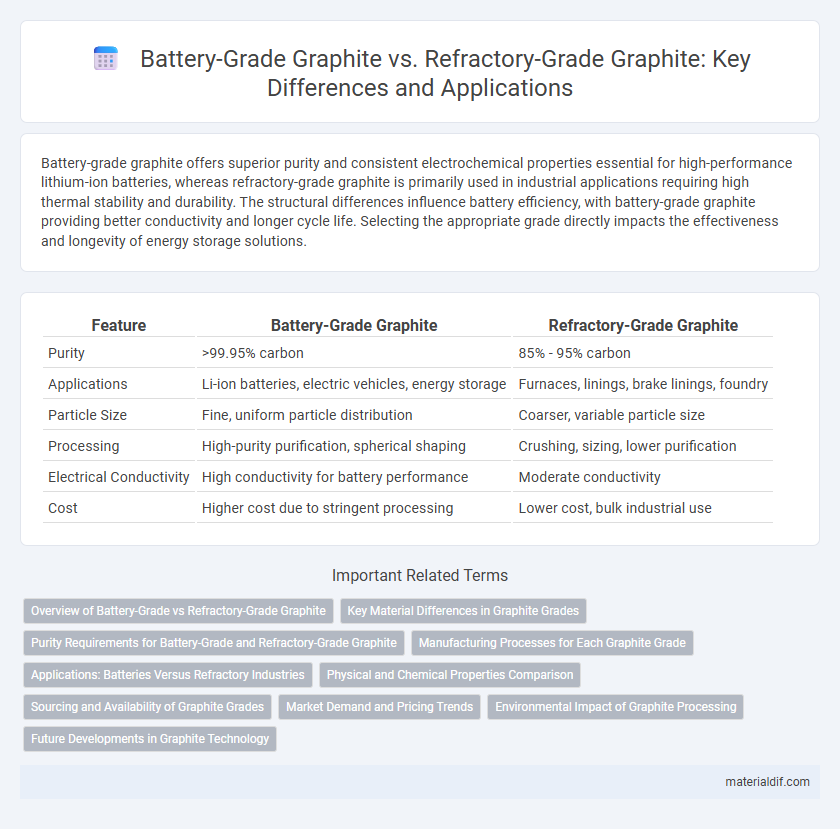
| Feature | Battery-Grade Graphite | Refractory-Grade Graphite |
|---|---|---|
| Purity | >99.95% carbon | 85% - 95% carbon |
| Applications | Li-ion batteries, electric vehicles, energy storage | Furnaces, linings, brake linings, foundry |
| Particle Size | Fine, uniform particle distribution | Coarser, variable particle size |
| Processing | High-purity purification, spherical shaping | Crushing, sizing, lower purification |
| Electrical Conductivity | High conductivity for battery performance | Moderate conductivity |
| Cost | Higher cost due to stringent processing | Lower cost, bulk industrial use |
Overview of Battery-Grade vs Refractory-Grade Graphite
Battery-grade graphite is characterized by high purity levels exceeding 99.95% carbon content, essential for use in lithium-ion battery anodes to ensure optimal performance and energy efficiency. Refractory-grade graphite, with lower purity typically around 85-95%, is primarily utilized for its thermal stability and resistance in high-temperature industrial applications such as furnace linings. The distinction between battery-grade and refractory-grade graphite lies in their structural and chemical properties tailored to meet the specific demands of energy storage versus thermal resistance industries.
Key Material Differences in Graphite Grades
Battery-grade graphite differs significantly from refractory-grade graphite in purity levels, with battery-grade requiring over 99.95% carbon content to ensure optimal performance in lithium-ion batteries. Refractory-grade graphite, commonly used in high-temperature applications, typically contains lower carbon purity around 85-95% but offers superior thermal resistance and structural integrity. The microstructure also varies; battery-grade graphite exhibits fine particle size and high crystallinity for efficient electron mobility, while refractory-grade has coarser grains suitable for heat-resisting molds.
Purity Requirements for Battery-Grade and Refractory-Grade Graphite
Battery-grade graphite requires a purity level exceeding 99.95% carbon content to ensure optimal performance in lithium-ion battery anodes, minimizing impurities that can affect conductivity and cycle life. Refractory-grade graphite typically has a lower purity threshold, around 85-95%, as it is used in high-temperature applications where perfect purity is less critical. The stringent purity requirements for battery-grade graphite drive advanced processing techniques such as chemical purification and floatation to achieve the necessary material quality.
Manufacturing Processes for Each Graphite Grade
Battery-grade graphite undergoes precise purification processes such as chemical leaching and high-temperature thermal purification to achieve purity levels above 99.95%, essential for lithium-ion battery anodes. In contrast, refractory-grade graphite is manufactured through high-temperature graphitization of carbon-rich materials like petroleum coke and coal tar pitch, focusing on structural strength and thermal resistance for industrial applications. The distinct manufacturing methods highlight the tailored purification and processing techniques that optimize graphite quality based on its intended use in energy storage or refractory industries.
Applications: Batteries Versus Refractory Industries
Battery-grade graphite is essential for lithium-ion batteries, providing high purity and stable electrochemical performance critical for electric vehicles and portable electronics. Refractory-grade graphite, with its high thermal stability and resistance to chemical corrosion, is primarily used in furnace linings, foundries, and metallurgical processes. The distinct physical and chemical properties of each grade tailor their applications to energy storage and high-temperature industrial environments, respectively.
Physical and Chemical Properties Comparison
Battery-grade graphite exhibits high purity levels exceeding 99.95% carbon content, low ash content, and superior electrical conductivity crucial for lithium-ion battery anodes. Refractory-grade graphite contains lower carbon content, higher impurities such as ash and sulfur, and possesses enhanced thermal stability and oxidation resistance suitable for high-temperature industrial applications. The physical properties differ with battery-grade graphite showing finer particle size and higher crystallinity, while refractory-grade graphite features larger grain size and greater mechanical strength to withstand extreme heat.
Sourcing and Availability of Graphite Grades
Battery-grade graphite primarily originates from high-purity natural flake deposits in countries such as China, Mozambique, and Canada, where advanced processing ensures low impurity levels critical for lithium-ion battery performance. Refractory-grade graphite, often sourced as synthetic graphite produced from petroleum coke or lower-grade natural graphite, is more widely available and less costly, meeting the demands of applications like steelmaking and foundry use. The limited geographic availability and stringent quality requirements of battery-grade graphite drive higher prices and supply chain complexities compared to the more abundant, industrial-grade refractory graphite.
Market Demand and Pricing Trends
Battery-grade graphite commands a premium price due to its high purity and critical role in lithium-ion battery production, driving strong market demand amid the electric vehicle industry's rapid growth. Refractory-grade graphite, used primarily in steel manufacturing and foundries, experiences more stable but lower demand linked to industrial cycles. Pricing trends show battery-grade graphite prices increasing sharply with electric vehicle adoption, while refractory-grade graphite prices remain relatively steady, influenced by traditional manufacturing activities.
Environmental Impact of Graphite Processing
Battery-grade graphite production demands extensive purification processes, resulting in higher energy consumption and increased emissions compared to refractory-grade graphite. Refractory-grade graphite, often sourced with fewer impurities, requires less intensive chemical treatment, leading to a lower environmental footprint. Sustainable advancements in battery-grade graphite processing aim to reduce toxic waste and greenhouse gases, enhancing the overall eco-friendliness of battery materials.
Future Developments in Graphite Technology
Battery-grade graphite, prized for its high purity and consistent particle size, is central to advancing lithium-ion battery efficiency and lifespan, driving ongoing innovations in coating and surface modification technologies. Refractory-grade graphite, essential for high-temperature industrial applications, is being enhanced through improved manufacturing methods that increase thermal stability and mechanical strength. Future developments focus on scalable, eco-friendly production processes and hybrid graphite materials to meet escalating demands in energy storage and industrial sectors.
Battery-Grade Graphite vs Refractory-Grade Graphite Infographic
 materialdif.com
materialdif.com