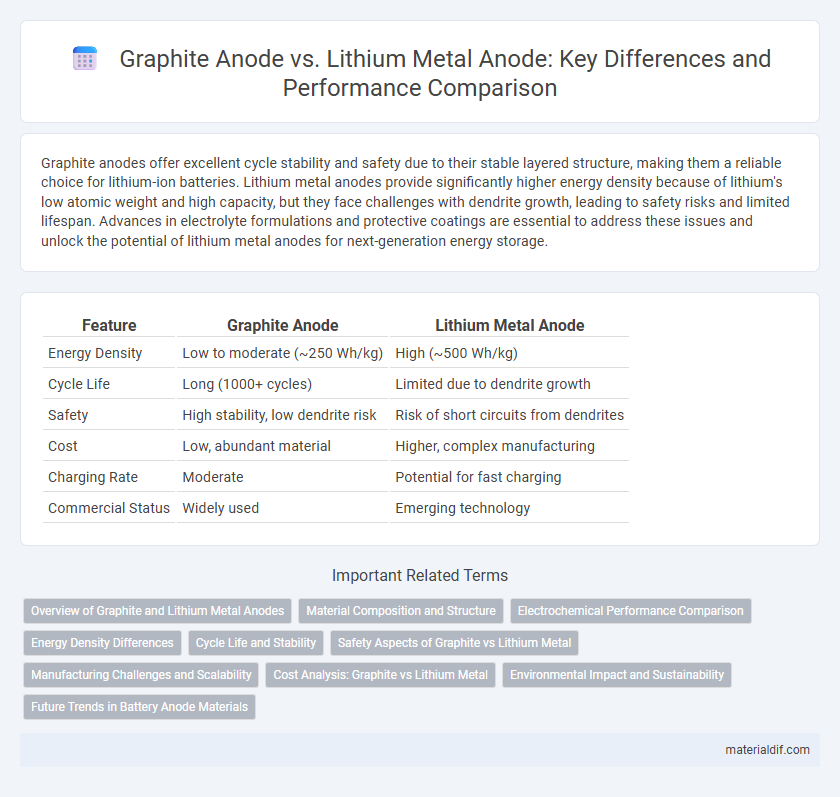
Graphite anodes offer excellent cycle stability and safety due to their stable layered structure, making them a reliable choice for lithium-ion batteries. Lithium metal anodes provide significantly higher energy density because of lithium's low atomic weight and high capacity, but they face challenges with dendrite growth, leading to safety risks and limited lifespan. Advances in electrolyte formulations and protective coatings are essential to address these issues and unlock the potential of lithium metal anodes for next-generation energy storage.
Table of Comparison
| Feature | Graphite Anode | Lithium Metal Anode |
|---|---|---|
| Energy Density | Low to moderate (~250 Wh/kg) | High (~500 Wh/kg) |
| Cycle Life | Long (1000+ cycles) | Limited due to dendrite growth |
| Safety | High stability, low dendrite risk | Risk of short circuits from dendrites |
| Cost | Low, abundant material | Higher, complex manufacturing |
| Charging Rate | Moderate | Potential for fast charging |
| Commercial Status | Widely used | Emerging technology |
Overview of Graphite and Lithium Metal Anodes
Graphite anodes consist of layered carbon structures that enable efficient lithium-ion intercalation, offering stability and long cycle life in lithium-ion batteries. Lithium metal anodes provide significantly higher theoretical capacity and energy density but face challenges like dendrite formation and limited cycling stability. The choice between graphite and lithium metal anodes involves balancing safety, capacity, and longevity for advanced battery applications.
Material Composition and Structure
Graphite anodes consist of layered carbon sheets with a hexagonal lattice, allowing lithium ions to intercalate between layers during charging, which provides structural stability and high cycle life. Lithium metal anodes are composed of pure lithium, offering significantly higher theoretical capacity due to lithium's high specific charge but suffer from dendrite formation caused by uneven lithium deposition. The graphite's composite carbon structure enhances safety and longevity, while lithium metal's elemental structure demands advanced materials engineering to mitigate short-circuit risks.
Electrochemical Performance Comparison
Graphite anodes offer stable electrochemical performance characterized by a specific capacity of about 372 mAh/g and excellent cycle stability, making them the industry standard for lithium-ion batteries. Lithium metal anodes deliver significantly higher specific capacity, approximately 3,860 mAh/g, and lower electrode potential, which can enhance energy density but pose challenges related to dendrite formation and cyclability. Comparative studies reveal that while lithium metal anodes have superior theoretical performance, graphite anodes maintain higher practical reliability and longevity under typical operating conditions.
Energy Density Differences
Graphite anodes typically offer energy densities around 250-300 Wh/kg, while lithium metal anodes can achieve significantly higher energy densities, reaching up to 400 Wh/kg or more. The higher theoretical capacity of lithium metal anodes, approximately 3,860 mAh/g compared to graphite's 372 mAh/g, directly contributes to this enhanced energy density. These differences make lithium metal anodes a promising choice for next-generation batteries aimed at maximizing energy storage and extending electric vehicle range.
Cycle Life and Stability
Graphite anodes exhibit superior cycle life and stability compared to lithium metal anodes due to their ability to intercalate lithium ions with minimal volume expansion, reducing mechanical stress during charge-discharge cycles. Lithium metal anodes, despite higher theoretical capacity, suffer from dendrite formation and significant volume changes that compromise cycle stability and safety. Advances in protective electrolytes and artificial SEI layers aim to enhance lithium metal anode stability but currently, graphite remains the benchmark for long-term battery cycling performance.
Safety Aspects of Graphite vs Lithium Metal
Graphite anodes exhibit superior safety profiles compared to lithium metal anodes due to their stable solid electrolyte interphase (SEI) formation, which mitigates dendrite growth and reduces the risk of short circuits and thermal runaway. Lithium metal anodes, while offering higher energy density, are prone to lithium dendrite formation that can puncture the separator, causing internal short circuits and compromising battery safety. The robust nature of graphite anodes contributes to enhanced cycle life and reduced fire hazards in lithium-ion battery applications.
Manufacturing Challenges and Scalability
Graphite anodes benefit from established manufacturing processes and supply chains, enabling scalable mass production with consistent quality control. Lithium metal anodes face significant challenges such as dendrite formation, complex electrolyte compatibility, and the need for advanced protective coatings, which complicate large-scale manufacturing. These technical hurdles limit lithium metal anode scalability compared to the more mature and commercially viable graphite anode technology.
Cost Analysis: Graphite vs Lithium Metal
Graphite anodes, widely used in lithium-ion batteries, offer a cost-effective solution due to abundant raw material availability and established manufacturing processes. Lithium metal anodes present higher energy density but entail significantly higher costs driven by complex production methods and safety-related material enhancements. Cost analysis reveals graphite anodes maintain a lower price per kilowatt-hour, making them economically favorable for large-scale battery applications despite lithium metal's potential performance advantages.
Environmental Impact and Sustainability
Graphite anodes offer greater environmental sustainability compared to lithium metal anodes due to their abundant natural availability and established recycling processes, reducing reliance on scarce lithium resources. The mining and processing of graphite have a lower carbon footprint and produce less hazardous waste than lithium extraction, which often involves environmentally damaging practices. Furthermore, advancements in synthetic graphite production are enhancing the sustainability profile by minimizing ecological disruption and improving material efficiency.
Future Trends in Battery Anode Materials
Graphite anodes dominate current lithium-ion batteries due to their stability, high cycle life, and cost-effectiveness, yet lithium metal anodes offer significantly higher energy density, paving the way for next-generation batteries. Research trends emphasize overcoming lithium metal challenges such as dendrite formation and limited cycle life through advanced solid electrolytes and protective coatings. Emerging materials like silicon-graphite composites and novel nanostructured lithium metal anodes are driving future innovations toward safer, higher-capacity energy storage solutions.
Graphite Anode vs Lithium Metal Anode Infographic
 materialdif.com
materialdif.com