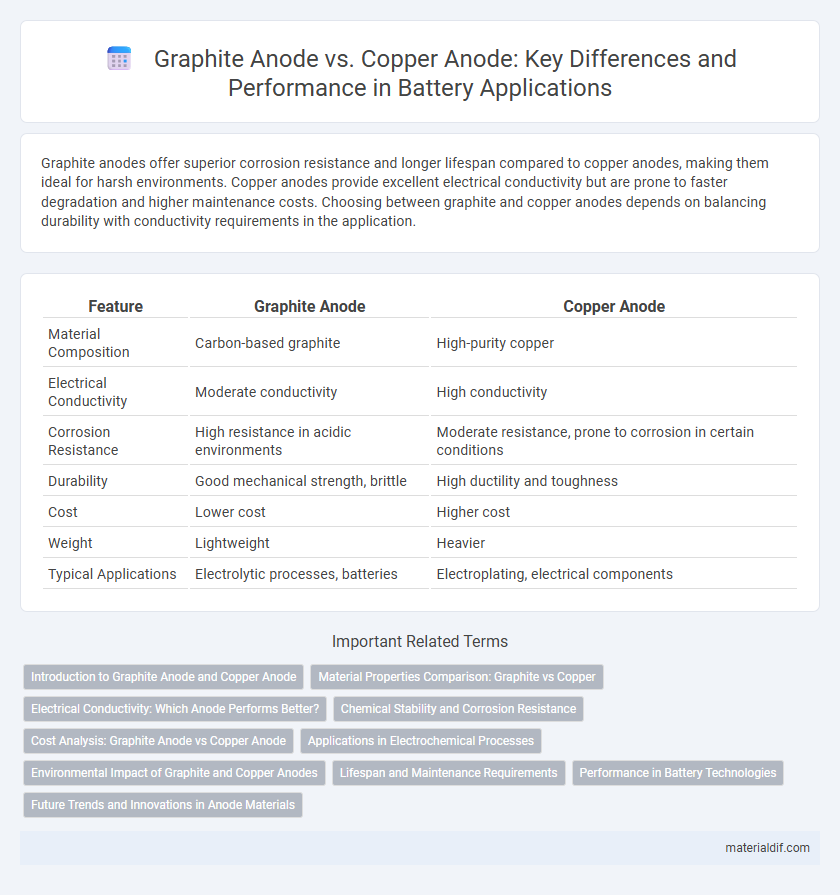
Graphite anodes offer superior corrosion resistance and longer lifespan compared to copper anodes, making them ideal for harsh environments. Copper anodes provide excellent electrical conductivity but are prone to faster degradation and higher maintenance costs. Choosing between graphite and copper anodes depends on balancing durability with conductivity requirements in the application.
Table of Comparison
| Feature | Graphite Anode | Copper Anode |
|---|---|---|
| Material Composition | Carbon-based graphite | High-purity copper |
| Electrical Conductivity | Moderate conductivity | High conductivity |
| Corrosion Resistance | High resistance in acidic environments | Moderate resistance, prone to corrosion in certain conditions |
| Durability | Good mechanical strength, brittle | High ductility and toughness |
| Cost | Lower cost | Higher cost |
| Weight | Lightweight | Heavier |
| Typical Applications | Electrolytic processes, batteries | Electroplating, electrical components |
Introduction to Graphite Anode and Copper Anode
Graphite anodes are widely used in electrochemical applications due to their excellent electrical conductivity, chemical stability, and resistance to corrosion, making them ideal for batteries and electrolysis processes. Copper anodes offer high electrical conductivity and mechanical strength but are more prone to corrosion and require protective coatings in many industrial applications. Selecting between graphite and copper anodes depends on the specific requirements of conductivity, durability, and chemical environment in the intended use.
Material Properties Comparison: Graphite vs Copper
Graphite anodes exhibit superior chemical stability and higher thermal resistance compared to copper anodes, making them ideal for high-temperature electrochemical applications. Graphite's low electrical conductivity relative to copper is offset by its excellent corrosion resistance and lightweight structure, which enhances durability and operational lifespan. Copper anodes offer significantly higher electrical conductivity but suffer from rapid oxidation and material degradation in harsh electrolytic environments, limiting their long-term performance.
Electrical Conductivity: Which Anode Performs Better?
Graphite anodes exhibit lower electrical conductivity compared to copper anodes due to graphite's semimetallic properties, resulting in higher resistivity and less efficient current flow in electrochemical cells. Copper anodes possess superior electrical conductivity, approximately 5.96 x 10^7 S/m, facilitating faster charge transfer and enhanced overall cell performance. In applications requiring rapid electron transfer and minimal resistance, copper anodes outperform graphite anodes in electrical conductivity.
Chemical Stability and Corrosion Resistance
Graphite anodes exhibit superior chemical stability and corrosion resistance compared to copper anodes, making them ideal for harsh electrochemical environments. Graphite's inert surface minimizes oxidation and degradation over time, while copper anodes are prone to corrosion through electrochemical reactions, limiting their lifespan. The higher resistance of graphite anodes to chemical attack enhances durability and reduces maintenance costs in industrial applications.
Cost Analysis: Graphite Anode vs Copper Anode
Graphite anodes generally present a lower upfront cost compared to copper anodes due to less expensive raw materials and simpler manufacturing processes. Over the lifecycle, graphite anodes offer longer service life and better resistance to corrosion, which reduces maintenance and replacement expenses, enhancing overall cost efficiency. Copper anodes, while having higher electrical conductivity, tend to incur greater total costs owing to material price volatility and frequent replacements in corrosive environments.
Applications in Electrochemical Processes
Graphite anodes excel in electrochemical processes requiring high corrosion resistance and thermal stability, commonly used in chlor-alkali production and electroplating. Copper anodes offer superior electrical conductivity, making them ideal for electrorefining and battery manufacturing. Both anode materials are selected based on specific process needs, balancing factors such as durability, efficiency, and cost.
Environmental Impact of Graphite and Copper Anodes
Graphite anodes exhibit a lower environmental footprint compared to copper anodes due to their higher recyclability and reduced energy consumption during production. Copper anode manufacturing involves intensive mining processes that significantly contribute to habitat destruction and greenhouse gas emissions. The use of graphite anodes supports sustainable battery production by minimizing toxic waste and promoting resource efficiency in energy storage applications.
Lifespan and Maintenance Requirements
Graphite anodes offer a longer lifespan compared to copper anodes due to their superior corrosion resistance and stability in electrochemical environments. Maintenance requirements for graphite anodes are typically lower, as they resist oxidation and scaling better than copper, reducing the frequency of replacements and cleaning. Copper anodes, while conductive, tend to degrade faster and require more frequent inspection and maintenance to prevent performance loss in industrial applications.
Performance in Battery Technologies
Graphite anodes exhibit superior electrochemical stability and higher lithium-ion intercalation capacity compared to copper anodes, leading to enhanced battery cycle life and energy density. Copper anodes typically suffer from significant volume expansion and lower conductivity, resulting in reduced performance and faster degradation in lithium-ion batteries. The intrinsic properties of graphite, including its layered structure and excellent electrical conductivity, make it the preferred choice for anodes in high-performance battery technologies.
Future Trends and Innovations in Anode Materials
Graphite anodes dominate current lithium-ion battery technology due to their high stability and cycle life, but ongoing research into copper anodes highlights potential breakthroughs in energy density and charging speed. Future trends emphasize hybrid anode materials combining graphite with copper or silicon to enhance conductivity and capacity while mitigating structural degradation. Innovations in nanostructuring and surface coatings aim to improve electrode performance, paving the way for next-generation batteries with faster charging and longer lifespan.
Graphite Anode vs Copper Anode Infographic
 materialdif.com
materialdif.com