Pyrolytic graphite offers superior thermal conductivity and anisotropic properties compared to polycrystalline graphite, making it ideal for high-performance thermal management applications. Polycrystalline graphite is composed of randomly oriented crystallites, resulting in isotropic characteristics and lower thermal conductivity but enhanced mechanical strength and chemical resistance. Choosing between pyrolytic and polycrystalline graphite depends on specific requirements for thermal conductivity, mechanical durability, and manufacturing costs.
Table of Comparison
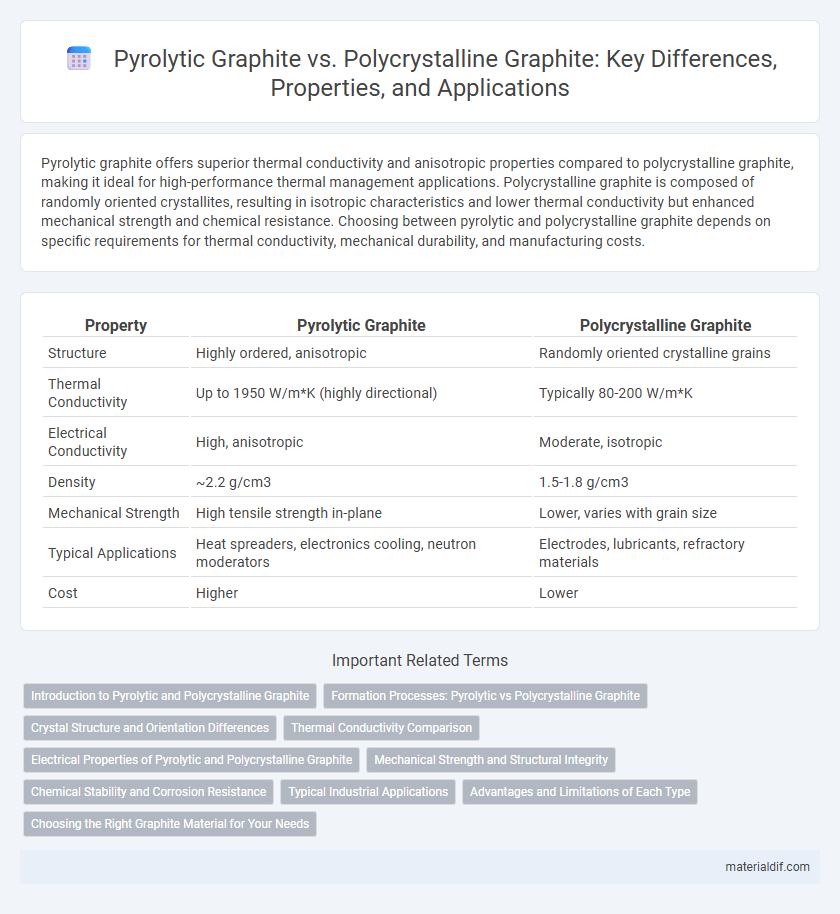
| Property | Pyrolytic Graphite | Polycrystalline Graphite |
|---|---|---|
| Structure | Highly ordered, anisotropic | Randomly oriented crystalline grains |
| Thermal Conductivity | Up to 1950 W/m*K (highly directional) | Typically 80-200 W/m*K |
| Electrical Conductivity | High, anisotropic | Moderate, isotropic |
| Density | ~2.2 g/cm3 | 1.5-1.8 g/cm3 |
| Mechanical Strength | High tensile strength in-plane | Lower, varies with grain size |
| Typical Applications | Heat spreaders, electronics cooling, neutron moderators | Electrodes, lubricants, refractory materials |
| Cost | Higher | Lower |
Introduction to Pyrolytic and Polycrystalline Graphite
Pyrolytic graphite is a highly ordered form of carbon produced by the thermal decomposition of hydrocarbons, characterized by its layered structure and exceptional anisotropic properties, such as high thermal conductivity and electrical conductivity along the basal plane. Polycrystalline graphite consists of numerous randomly oriented crystallites, resulting in isotropic mechanical and thermal properties with lower thermal conductivity compared to pyrolytic graphite. Both materials find extensive applications in high-temperature environments, electrochemical cells, and as components in advanced electronics, distinguished by their structural and performance differences.
Formation Processes: Pyrolytic vs Polycrystalline Graphite
Pyrolytic graphite forms through chemical vapor deposition, where hydrocarbons decompose at high temperatures to create highly ordered, layered graphite structures with anisotropic properties. In contrast, polycrystalline graphite results from the sintering and graphitization of coke and pitch materials, producing randomly oriented crystallites with isotropic characteristics. The distinct formation processes directly influence their microstructure, thermal conductivity, and mechanical strength.
Crystal Structure and Orientation Differences
Pyrolytic graphite exhibits a highly ordered crystal structure with well-aligned graphene layers stacked in a hexagonal arrangement, resulting in strong anisotropy and superior thermal conductivity along the basal plane. Polycrystalline graphite consists of randomly oriented graphite crystallites, leading to less uniform crystal orientation and isotropic properties with lower thermal and electrical conductivity. The distinct crystal structure and layer alignment in pyrolytic graphite make it ideal for applications requiring directional heat dissipation and mechanical strength.
Thermal Conductivity Comparison
Pyrolytic graphite exhibits significantly higher thermal conductivity, reaching up to 1950 W/m*K along its basal plane, compared to polycrystalline graphite, which typically ranges around 100-400 W/m*K due to its heterogeneous grain structure. The anisotropic nature of pyrolytic graphite enables efficient heat dissipation in specific directions, making it ideal for high-performance thermal management applications. In contrast, the isotropic thermal properties of polycrystalline graphite result in more uniform but generally lower thermal conductivity.
Electrical Properties of Pyrolytic and Polycrystalline Graphite
Pyrolytic graphite exhibits superior electrical conductivity compared to polycrystalline graphite due to its highly ordered layered structure, which facilitates electron mobility along the basal planes. In contrast, polycrystalline graphite displays more isotropic electrical behavior with lower conductivity, attributed to grain boundaries and random crystal orientations that scatter charge carriers. The anisotropic electrical properties of pyrolytic graphite make it ideal for applications requiring high in-plane conductivity and thermal management.
Mechanical Strength and Structural Integrity
Pyrolytic graphite exhibits superior mechanical strength and structural integrity due to its highly ordered, layered crystal structure, which enhances its rigidity and resistance to deformation under stress. In contrast, polycrystalline graphite has a more disordered grain structure with numerous grain boundaries, leading to lower mechanical strength and increased susceptibility to crack propagation. The anisotropic properties of pyrolytic graphite make it ideal for applications requiring high strength and durability, while polycrystalline graphite is better suited for environments where isotropic thermal and electrical properties are prioritized over mechanical performance.
Chemical Stability and Corrosion Resistance
Pyrolytic graphite exhibits superior chemical stability and corrosion resistance due to its highly ordered layered structure and strong covalent bonding, making it resistant to oxidation and chemical attack even at elevated temperatures. In contrast, polycrystalline graphite, composed of randomly oriented crystallites with more grain boundaries, is more susceptible to chemical degradation and corrosive environments. These differences make pyrolytic graphite ideal for applications requiring high durability in aggressive chemical or thermal conditions.
Typical Industrial Applications
Pyrolytic graphite, with its high thermal conductivity and anisotropic properties, is typically used in electronic heat sinks, thermal management systems, and specialized aerospace components requiring efficient heat dissipation. Polycrystalline graphite, characterized by isotropic mechanical strength and chemical resistance, is commonly employed in electrodes for electric arc furnaces, crucibles, and mechanical seals in the metallurgical and chemical industries. These distinct properties drive their use in applications where thermal performance or structural durability is prioritized.
Advantages and Limitations of Each Type
Pyrolytic graphite offers superior thermal conductivity and anisotropic electrical properties, making it ideal for high-performance heat sinks and electronic applications, but it is limited by higher production costs and brittleness compared to polycrystalline graphite. Polycrystalline graphite provides enhanced mechanical strength and isotropic properties due to its randomly oriented grains, resulting in cost-effective manufacturing and robustness, though it has lower thermal conductivity and electrical anisotropy. Both materials serve distinct industrial applications based on their thermal, electrical, and mechanical characteristics, balancing performance needs and economic factors.
Choosing the Right Graphite Material for Your Needs
Pyrolytic graphite offers superior thermal conductivity and anisotropic properties, making it ideal for applications requiring efficient heat dissipation and directional conductivity. Polycrystalline graphite, characterized by its isotropic structure and mechanical strength, excels in wear resistance and structural stability for industrial uses. Selecting the right graphite depends on thermal performance requirements, mechanical durability, and specific application environments.
Pyrolytic Graphite vs Polycrystalline Graphite Infographic
 materialdif.com
materialdif.com