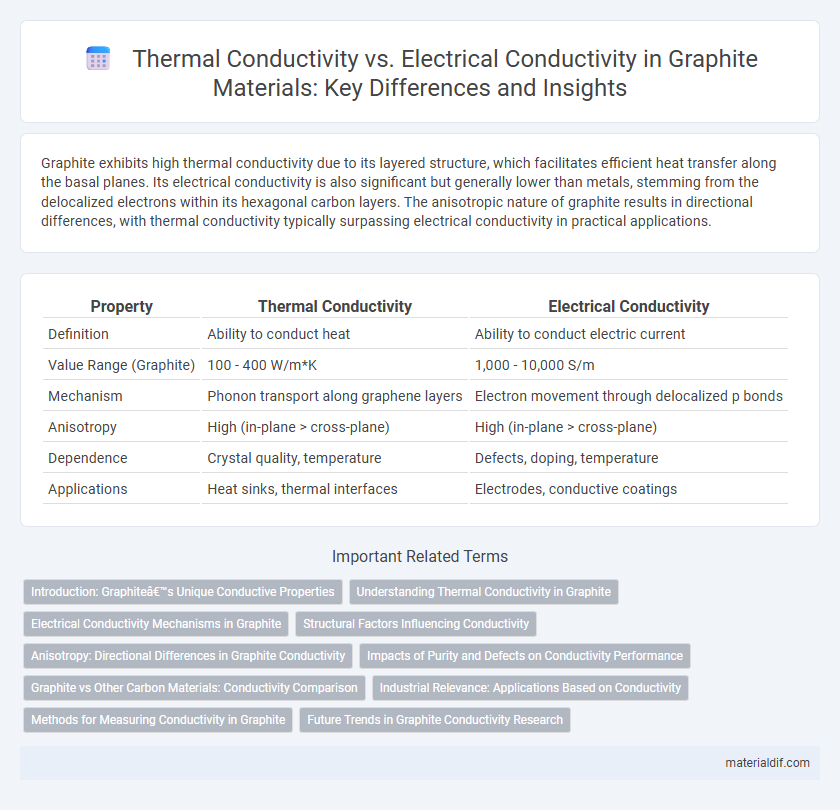
Graphite exhibits high thermal conductivity due to its layered structure, which facilitates efficient heat transfer along the basal planes. Its electrical conductivity is also significant but generally lower than metals, stemming from the delocalized electrons within its hexagonal carbon layers. The anisotropic nature of graphite results in directional differences, with thermal conductivity typically surpassing electrical conductivity in practical applications.
Table of Comparison
| Property | Thermal Conductivity | Electrical Conductivity |
|---|---|---|
| Definition | Ability to conduct heat | Ability to conduct electric current |
| Value Range (Graphite) | 100 - 400 W/m*K | 1,000 - 10,000 S/m |
| Mechanism | Phonon transport along graphene layers | Electron movement through delocalized p bonds |
| Anisotropy | High (in-plane > cross-plane) | High (in-plane > cross-plane) |
| Dependence | Crystal quality, temperature | Defects, doping, temperature |
| Applications | Heat sinks, thermal interfaces | Electrodes, conductive coatings |
Introduction: Graphite’s Unique Conductive Properties
Graphite exhibits exceptional thermal conductivity due to its layered crystal structure, allowing efficient heat transfer along the basal planes. Its electrical conductivity is also notable, attributed to delocalized electrons within the hexagonal carbon layers, enabling rapid electron mobility. These dual conductive properties make graphite an essential material in applications requiring both thermal management and electrical conduction.
Understanding Thermal Conductivity in Graphite
Thermal conductivity in graphite materials is primarily attributed to the strong covalent bonding within the hexagonal layers, allowing efficient phonon transport along the basal planes. Unlike electrical conductivity, which is dominated by delocalized electrons facilitating charge transfer, thermal conductivity relies on lattice vibrations and exhibits anisotropy, with much higher values parallel to the graphene layers compared to the perpendicular direction. The unique layered structure of graphite results in high in-plane thermal conductivity, typically ranging from 100 to 400 W/m*K, making it an exceptional material for heat dissipation applications.
Electrical Conductivity Mechanisms in Graphite
Graphite exhibits high electrical conductivity due to its layered structure, where delocalized p-electrons move freely within the graphene planes, enabling efficient electron transport. Thermal conductivity in graphite is primarily phonon-driven, involving lattice vibrations perpendicular to electrical conduction pathways, explaining the disparity between thermal and electrical conductivity values. The anisotropic nature of graphite results in superior in-plane electrical conductivity compared to its cross-plane conductivity, highlighting the significance of electron mobility in the basal plane.
Structural Factors Influencing Conductivity
Graphite's thermal conductivity is predominantly governed by its layered hexagonal crystal structure, allowing efficient phonon transport along graphene planes, while electrical conductivity benefits from delocalized pi electrons facilitating electron mobility within these layers. Variations in layer stacking order, crystallite size, and defect density directly impact both thermal and electrical conduction by altering phonon scattering and electron hopping mechanisms. Anisotropy inherent in graphite creates a significant disparity between in-plane and cross-plane conductivities, emphasizing the role of structural factors in determining overall material performance.
Anisotropy: Directional Differences in Graphite Conductivity
Graphite exhibits significant anisotropy in both thermal and electrical conductivity, with conductivity along the basal planes (parallel to graphene layers) being much higher than perpendicular to these planes. Thermal conductivity in the basal plane can reach values up to 2000 W/m*K, whereas cross-plane conductivity often falls below 10 W/m*K, demonstrating extreme directional dependence. Electrical conductivity follows a similar trend, with in-plane conductivity orders of magnitude greater than through-plane conductivity due to the layered crystal structure and strong covalent bonding within planes contrasted with weak van der Waals forces between layers.
Impacts of Purity and Defects on Conductivity Performance
Graphite's thermal conductivity is higher than its electrical conductivity due to the anisotropic bonding structure, with purity levels directly enhancing electron and phonon transport by minimizing scattering sites. Defects, such as vacancies and grain boundaries, disrupt the crystalline lattice, significantly reducing both thermal and electrical conductivity, though electrical performance is more sensitive to such imperfections. Optimizing graphite purity and minimizing structural defects are critical to maintaining superior conductivity performance in high-demand applications like heat sinks and electrodes.
Graphite vs Other Carbon Materials: Conductivity Comparison
Graphite exhibits high thermal conductivity ranging from 100 to 400 W/m*K, surpassing many other carbon materials like amorphous carbon and carbon fibers, which typically show lower thermal conductivity values below 50 W/m*K. Its electrical conductivity, approximately 10^4 S/m along the basal plane, is superior to amorphous carbon but lower than highly ordered pyrolytic graphite (HOPG) and graphene variants that reach conductivities up to 10^6 S/m. This unique combination of moderate-to-high thermal and electrical conductivities makes graphite a preferred material in applications demanding efficient heat dissipation alongside decent electrical conduction.
Industrial Relevance: Applications Based on Conductivity
Graphite exhibits high thermal conductivity, typically around 120-165 W/m*K, which makes it ideal for heat dissipation in industrial applications such as heat sinks and thermal management systems. Its electrical conductivity, often exceeding 10^4 S/m, enables its use in electrodes, batteries, and conductive coatings where efficient electrical flow is critical. The combination of thermal and electrical conductivity in graphite materials is crucial for advanced manufacturing processes in electronics, energy storage, and high-temperature industrial environments.
Methods for Measuring Conductivity in Graphite
Thermal conductivity in graphite is commonly measured using the laser flash analysis (LFA) method, where a short laser pulse heats one side of the sample and temperature rise is monitored to calculate thermal diffusivity. Electrical conductivity measurement in graphite typically employs the four-point probe technique, which minimizes contact resistance errors by using separate pairs of electrodes for current injection and voltage measurement. Accurate conductivity characterization in graphite requires controlling sample purity, orientation of graphene layers, and temperature conditions during testing to ensure reliable and reproducible results.
Future Trends in Graphite Conductivity Research
Graphite materials exhibit a unique interplay between thermal conductivity and electrical conductivity, driven by their layered crystal structure and electron mobility. Emerging research focuses on manipulating defects and doping levels to enhance the anisotropic thermal conductivity while maintaining or improving electrical conductivity for advanced electronic and thermal management applications. Future trends highlight the integration of graphene derivatives and hybrid composites to optimize conductivity parameters for next-generation energy storage, flexible electronics, and high-performance thermal interface materials.
Thermal Conductivity vs Electrical Conductivity (in Graphite Materials) Infographic
 materialdif.com
materialdif.com